BLD Insights
Applications of Diazirine in Chemical Biology
05 April 2022
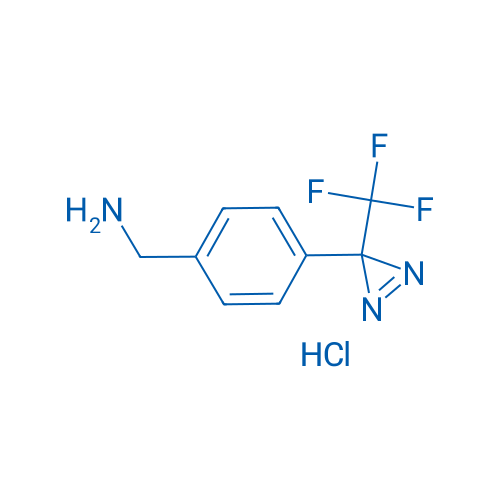
4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzylamine Hydrochloride
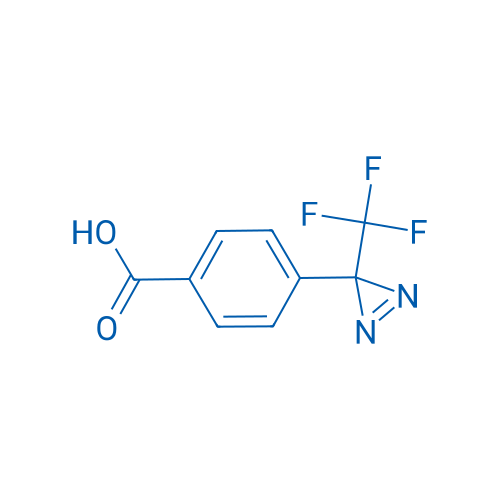
4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzoic Acid
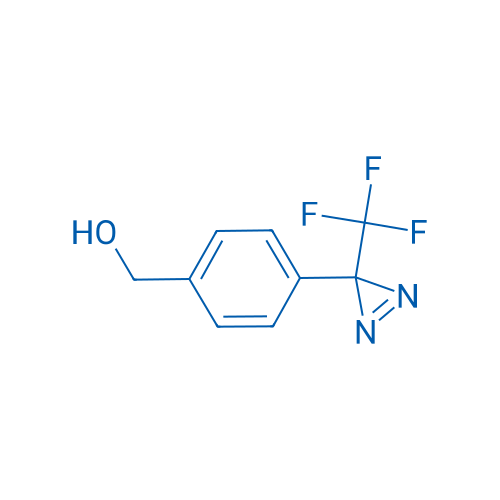
4-[3-(Trifluoromethyl)-3H-diazirin-3-yl]benzyl Alcohol
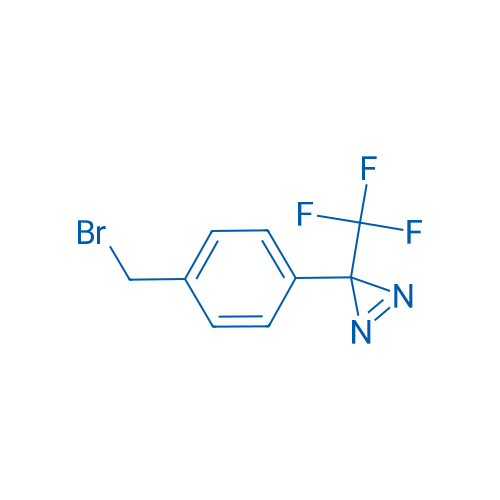
3-(4-(Bromomethyl)phenyl)-3-(trifluoromethyl)-3H-diazirine
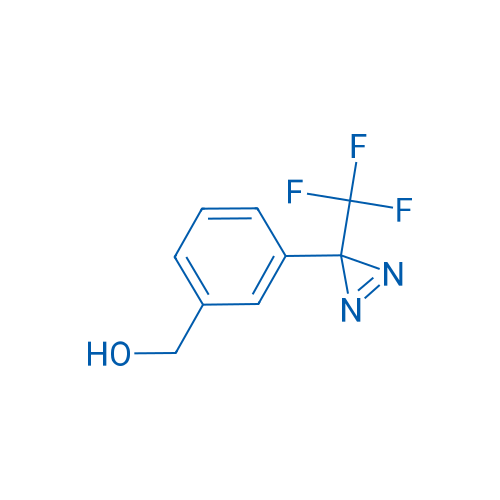
(3-(3-(Trifluoromethyl)-3H-diazirin-3-yl)phenyl)methanol
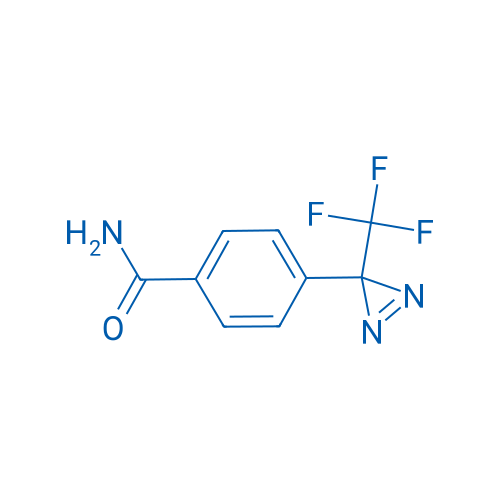
4-(3-(Trifluoromethyl)-3H-diazirin-3-yl)benzamide
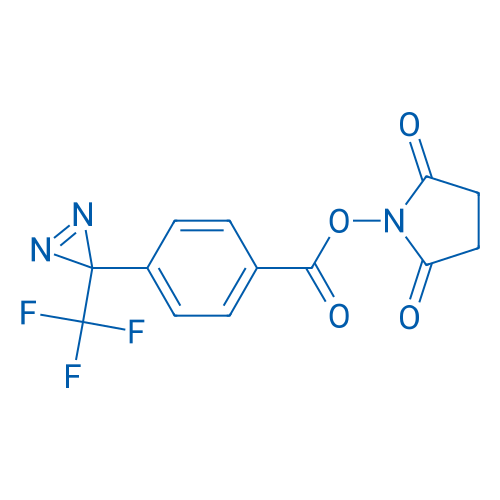
2,5-Dioxopyrrolidin-1-yl 4-(3-(trifluoromethyl)-3H-diazirin-3-yl)benzoate
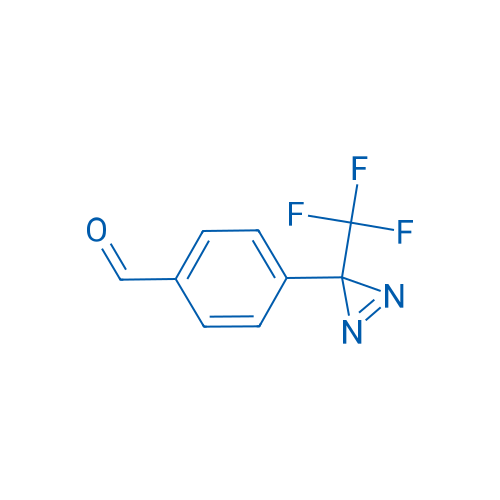
4-(3-(Trifluoromethyl)-3H-diazirin-3-yl)benzaldehyde
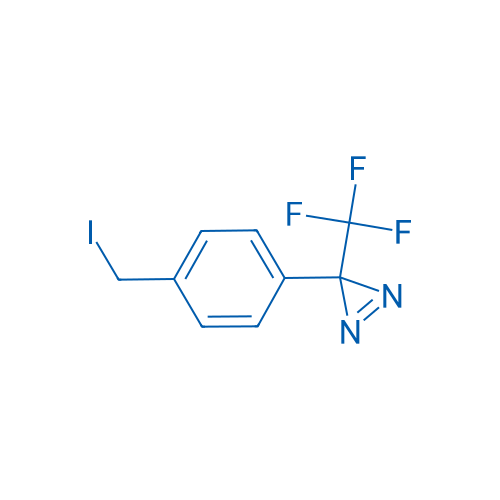
3-(4-(Iodomethyl)phenyl)-3-(trifluoromethyl)-3H-diazirine
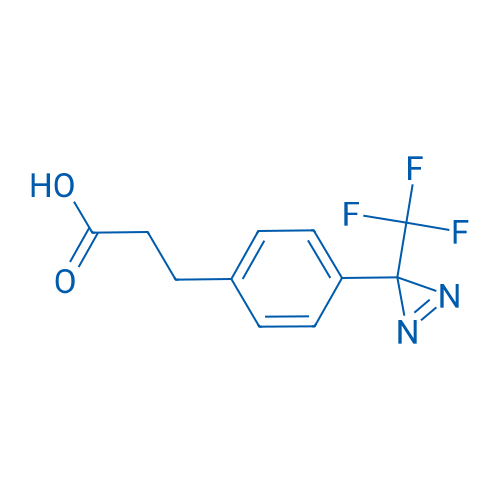
3-(4-(3-(Trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid
Background
Introducing targeted covalent ligands (fluorophores, isotopic labels, spin labels, etc.) to biological molecules has enhanced the understanding of donor-acceptor interaction and other fundamental bio-questions. Photoaffinity labeling (PAL)[1] was developed as an efficient method to realize this goal above, which appeared originally in the 1960s. Three major types of PAL groups are commonly used in PAL: benzophenones, arylazides, and diazirines[2]. Modified PAL probes were used to study donor(ligand)–acceptor interactions; identify the location of an enzyme inhibitor; define unknown bio-molecules, or distinguish amino acid residues, which helped a lot to investigate the structure-relationship, mechanism of action, and so on in vivo.
Mechanism of PALs
The general mechanism of PALs[2,3] is shown in Figure 1. Firstly, PAL probes recognize correlated protein(marked as Receptor in Figure 1) specifically via their molecular ligand(marked as LIG in Figure 1). Then, PAL probes occur photochemical reaction under UV irradiation, which produce highly reactive species that can covalently conjugate to the target protein. The probe-receptor complex is formed by this process. Finally, PAL probes usually possess a reporting group such as terminal alkyne which enables the complex bioorthogonally link to a tag(marked as TAG in Figure 1, eg, biotin, a fluorescent moiety, and radioactive labeling) for rapid detection and isolation of the proteins.
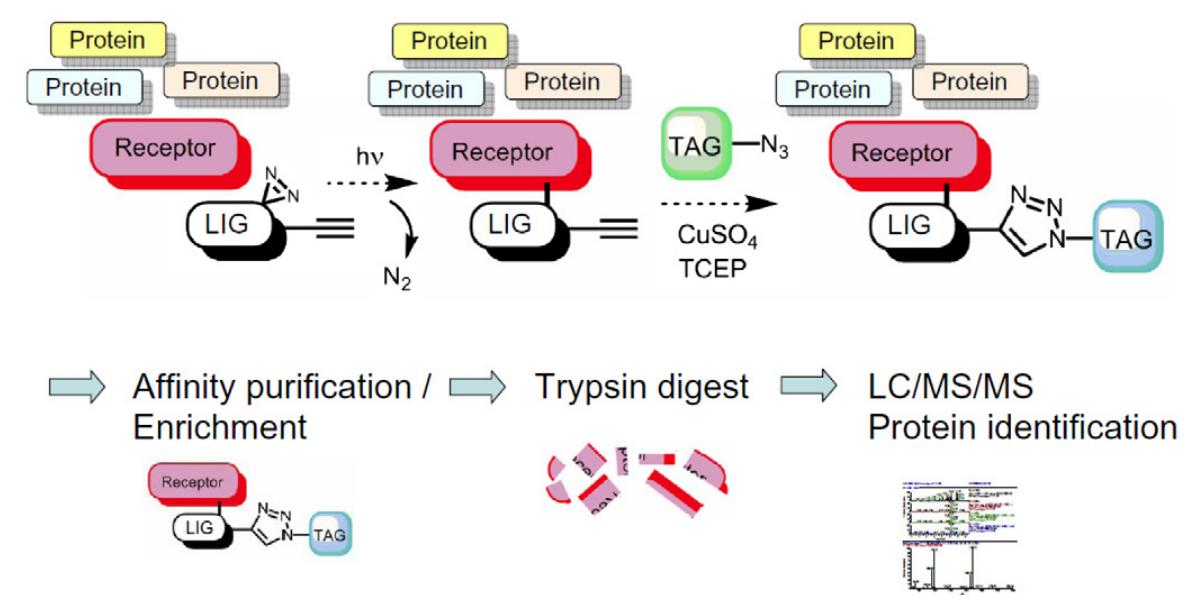
Figure 1: The general mechanism of PALs
The development of diazirine based photoaffinity labeling
In 1973, Smith and Knowles[4] developed the first PAL probe with a diazirine moiety. The advantages of diazirine compared with other types were listed below: 1. Activating the PAL probes with 350-360 nm UV light that is comparatively biocompatible; 2. short photo-irradiation time was needed; 3. The generated carbene species with high activity would quickly react with a biomolecule. In 1980, Brunner[5] developed 3-phenyl-3-(trifluoromethyl)diazirine (TPD) for suppressing diazo isomerization which lowers the PAL efficiency. In addition, they are easy to be derived due to chemically stable properties, hence a series of TPD-based probes have been developed[6-11].
Hot research fields
1. Propargyl diazirine based PAL probes
Propargyl diazirine-based PAL probes were widely used recently because the introduction of a minimalist alkynyl tag is convenient for click chemistry in biosystems. Generally, the aim of the click reaction is to conjugate with reporting tags such as biotin, then realize rapid enrichment and pull-down. Figure 2 shows a work related to propargyl diazirine-based PAL probes derived from rapamycin which is a famous mTOR(Mammalian target of rapamycin) immunosuppressant through ternary complex formation with FKBP12(FK506 binding protein 12). Analysis of the FKBP12–rapamycin–FRB ternary complex by PAL and MS yielded the first binding site hotspot map of a complex macrocyclic natural product. Data obtained with photo-rapamycin provide a 5.0 Å minimum distance restraint and a 9.0 Å predicted labeling radius for interpretation of binding site maps. The measurement may be broadly useful in the interpretation of binding site measurements from PAL[12].
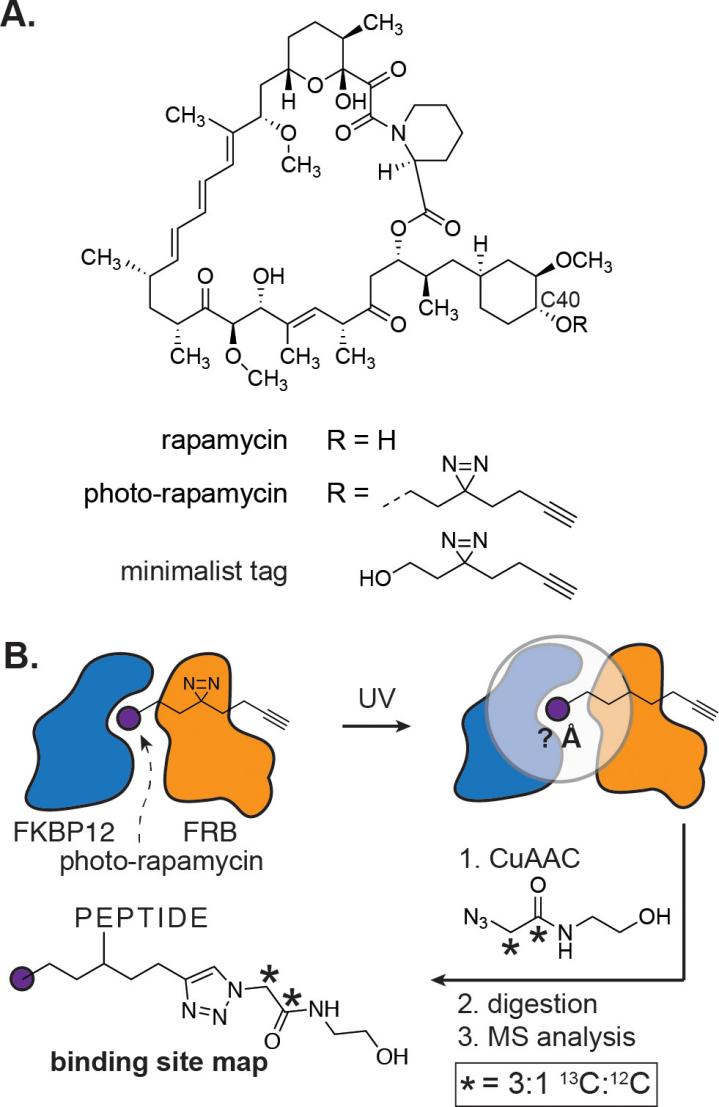
Figure 2: Structural characterization of small molecule binding site hotspots is enabled by phto-rapamycin
2. Aliphatic diazirine used in the unnatural amino acid synthesis
The unnatural amino acid containing Aliphatic diazirine can be used to prepare photo-active protein. Human caseinolytic protease P(hClpP) is a highly conserved serine protease found in the mitochondrial matrix and is an important part of the mitochondrial protein quality control system. Approaches for profiling protease substrates are critical for defining protease functions but remain challenging tasks. For this, as Figure 3 shows, the diazirine-bearing non-canonical amino acid DiazK is incorporated site-specifically at selected positions within the inner proteolytic chamber of hClpP and trapped hClpP-substrates are enriched and identified via mass spectrometry[13].
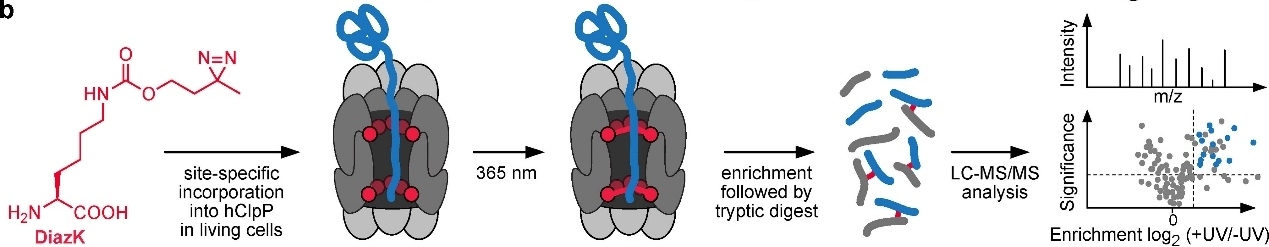
Figure 3: Substrate profiling of hClpP via a site-specific photo-crosslinking approach
3. TPD used in traceless immobilization of analytes
For the excellent chemical stability and biocompatibility, TPD derivatives have been favored by scientists. MALDI-TOF(Matrix-Assisted Laser Desorption/ Ionization Time of Flight) is well suited for characterizing biological activities based on the high throughput and quick analysis. In Figure 4, M. Mrksich group developed Au-S based self-assembled monolayer to assist MALDI-TOF in the detection of analytes that are either first immobilized to the monolayer and treated with an enzyme, or treated with the enzyme in a solution-phase reaction and subsequently immobilized to the monolayer prior to analysis by mass spectrometry. Because the TPD is able to trap a vast amount of analytes without pre-modifying a specific functional group, such as warfarin shown in Fig 4, it broadens the application range of MALDI-TOF[14].
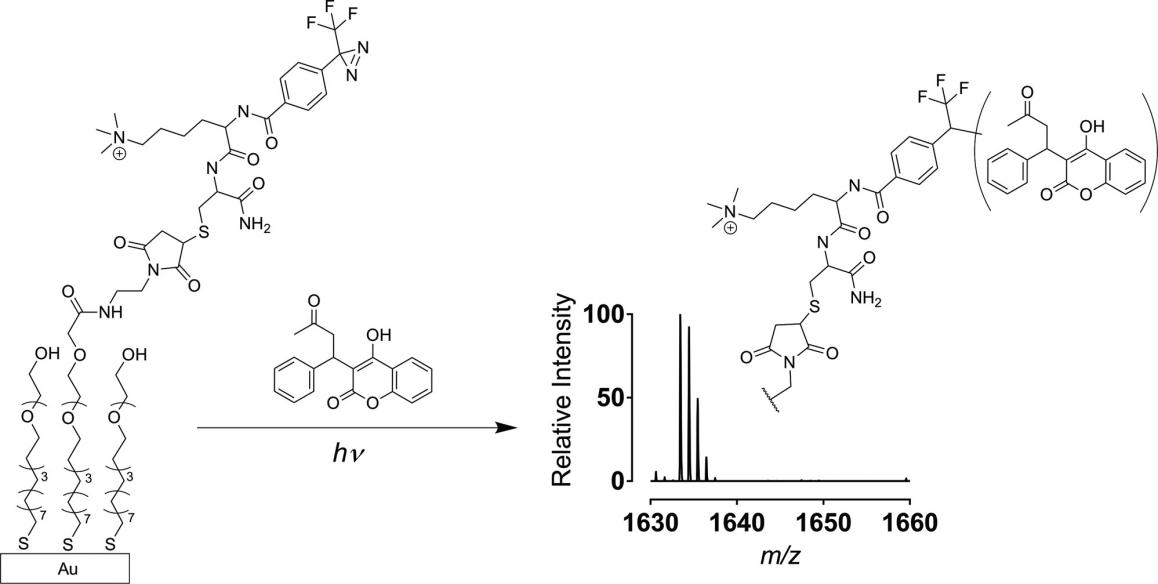
Figure 4: Traceless immobilization of analytes for high-throughput experiments with MS
References
[1]Singh, A.; Thornton, E. R.; Westheimer, F. H. J. Biol. Chem. 1962, 237, 3006.
[2]M. Hashimoto, Y. Hatanaka. Eur. J. Org. Chem. 2008, 2513–2523.
[3]L. Dubinsky et al. Bioorg. Med. Chem. 2012, 20, 554–570.
[4]Smith, R. A. G.; Knowles, J. R. J. Am. Chem. Soc. 1973, 95, 5072.
[5]Brunner, J.; Senn, H.; Richards, F. M. J. Biol. Chem. 1980,255, 3313-3318.
[6]T. Kinoshita, A. Cano-Delgado, H. Seto , S. Hiranuma, S. Fujioka, S Yoshida, J. Chory Nature 2015, 433, 167-171.
[7]G.-L. Chee et al. Bioorg. Med. Chem. 2010, 18, 830–838.
[8]Lee, J. S.; Han, S. Y.; Kim, M. S.; Yu, C. M.; Kim, M. H.; Kim, S. H.; Min, Y. K.; Kim, B. T. Bioorg. Med. Chem. Lett. 2006, 16, 4733.
[9]TPD derivatives of galactosylceramide. M. Hashimoto, Y. Hatanaka, K. Nabeta, Bioorg. Med. Chem. Lett. 2002, 12, 89–91.
[10]a) Brunner, J.; Semenza, G. Biochemistry 1981, 20, 7174. Kakutani, N.; Murai, M.; Sakiyama, N.; Miyoshi, H. Biochemistry 2010, 49, 4794; b)Murai, M.; Ishihara, A.; Nishioka, T.; Yagi, T.; Miyoshi, H. Biochemistry 2007, 46, 6409; c) Pinkse, M. W. H.; Rijkers, D. T. S.; Dostmann, W. R.; Heck, A. J. R. J. Biol. Chem. 2009, 284, 16354.
[11]Hatanaka, Y.; Hashimoto, M. Photoaffinity Labeling for Structural Probing within Protein; Springer Japan: Tokyo, 2017.
[12]H. Flaxman, C. Chang, H. Wu, C. Nakamoto, C. Woo, J. Am. Chem. Soc. 2019, 141, 30, 11759–11764.
[13]T. Nguyen, T. F. Gronauer, T. Nast-Kolb, S. Sieber, K. Lang, Angew. Chem. Int. Ed. 2022, 61, e202111085.
[14]K. Helal, A. Alamgir, E. Berns, M. Mrksich, J. Am. Chem. Soc. 2018, 140, 26, 8060–8063.