BLD Insights
Topoisomerases: the "scissor hand" of DNA repair
26 May 2022
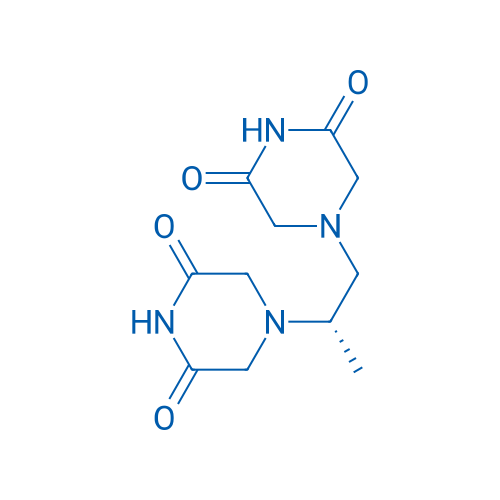
4,4'-[(1S)-1-Methyl-1,2-ethanediyl]bis-2,6-piperazinedione
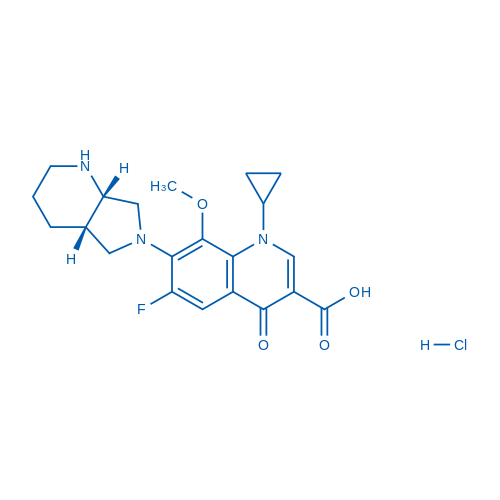
1-Cyclopropyl-6-fluoro-7-((4aS,7aS)-hexahydro-1H-pyrrolo[3,4-b]pyridin-6(2H)-yl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
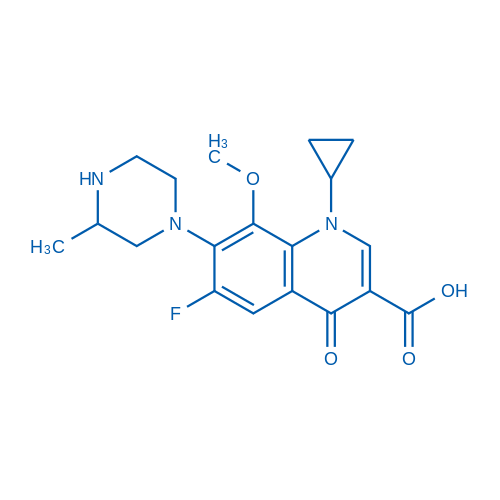
1-Cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
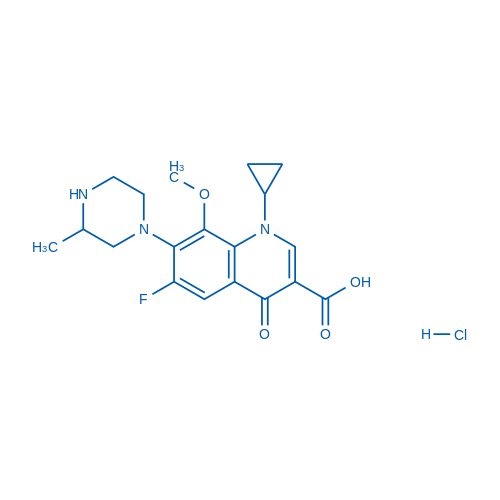
1-Cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
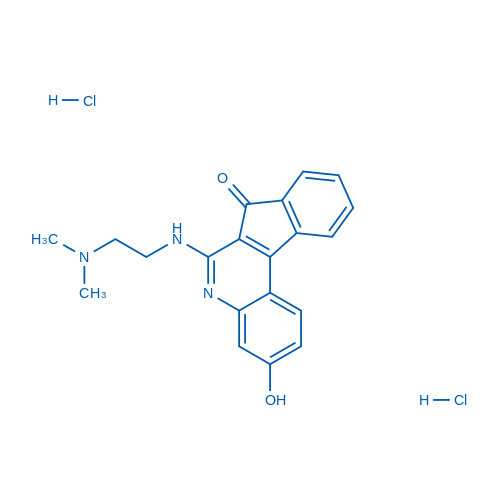
6-((2-(Dimethylamino)ethyl)amino)-3-hydroxy-7H-indeno[2,1-c]quinolin-7-one dihydrochloride
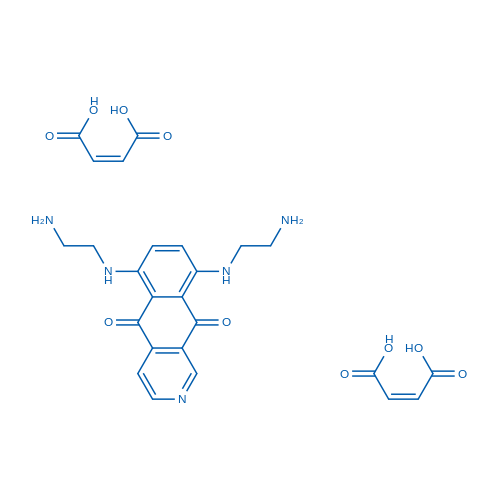
6,9-Bis((2-aminoethyl)amino)benzo[g]isoquinoline-5,10-dione dimaleate
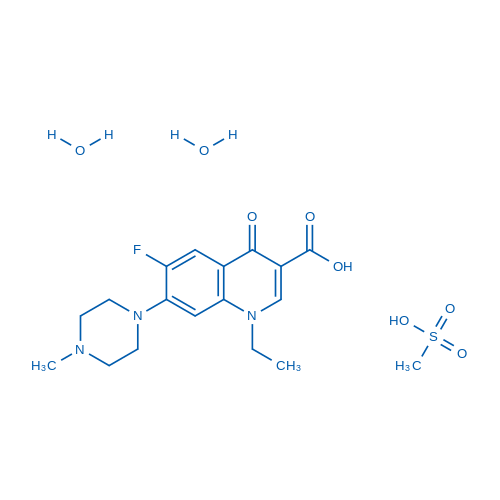
1-Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid mesylate dihydrate
In organisms, DNA generally exists stably in the form of double strand. In the process of DNA replication, the first step is to unwind the double strand into a single strand. However, just like two long ropes entangled together, when unwinding, some places will accidentally tie a dead knot, at this time, a "scissor hand" is needed to untie it, and the "scissor hand" in the cell is the topoisomerase. Topoisomerase can untie the DNA that has been knotted, and then reconnect it.
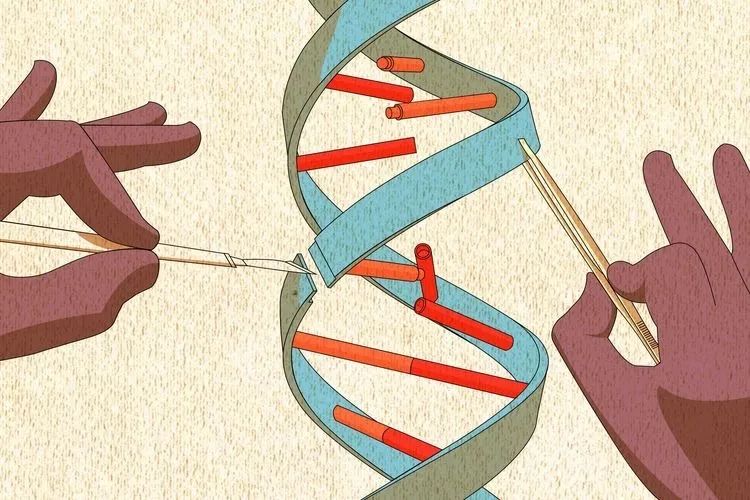
Figure 1. Function of Topoisomerase[1]
Topoisomerase
Topoisomerases are essential and ubiquitous enzymes that regulate DNA topology by making temporary single- (type I) or double-strand DNA breaks (type II) [2]. The DNA double helix needs to be unwound for processes such as DNA replication or transcription to take place, giving rise to the accumulation of positive supercoils ahead of transcription bubbles and replication forks, and negative supercoils or pre-catenanes behind. Topoisomerases relax supercoiled DNA and decatenate DNA to allow DNA transcription and replication to take place and are essential for genome stability [3]. While the creation of temporary double-strand breaks is necessary for the function of type II topoisomerases, it is potentially hazardous for the cell.
There are topoisomerase I (Top I) and topoisomerase II (Top II) in mammals, which act on single-stranded DNA and double-stranded DNA, respectively. Top I includes three subtypes IA, IB and IC; Top II includes two subtypes IIA and IIB[2][4]. Type IIA is found in bacteria and eukaryotes, whereas IIB was discovered in archaea and more recently in plants and plasmodial parasites. While lower eukaryotes and prokaryotes, such as bacteria, viruses and unicellular yeast, they only have Top I but not Top II.
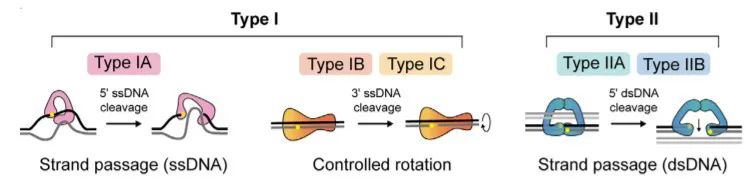
Figure 2. Topoisomerase Types[5]
Mechanism of topoisomerase action
Topoisomerase I is an ATP-independent enzyme that does not require ATP molecules for energy during the reaction. After topoisomerase I cleaves single-stranded DNA, it non-covalently forms a temporary easily dissociable complex intermediate (enzyme-DNA cleavage complex) with the terminal DNA fragment, and the nick reconnects after the stress is released. Topoisomerase II is an ATP-dependent enzyme that requires two ATP molecules per reaction. Topoisomerase II acts on double-stranded DNA, cutting and reconnecting it. This reaction is reversible, and this process is mainly to release the positive supercoiling pressure of eukaryotic DNA[1]. They catalyze the mutual conversion between the supercoiled state and the uncoiled state of DNA molecules on the basis of the double helix, and do not change the composition, number and sequence of bases.
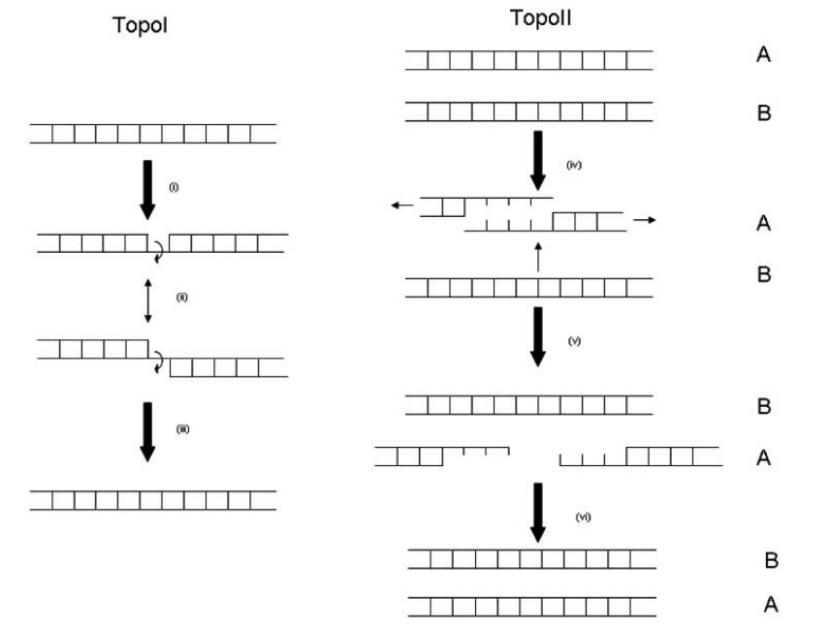
Figure 3. Schematic Diagram of Topoisomerase Action[6]
Topoisomerase inhibitors
Compared with normal cells, the content and activity of topoisomerase in tumor cells are significantly increased, so topoisomerase is an excellent target for antitumor drugs. Both Top I and Top II inhibitors have similar mechanisms of action, and can covalently form stable enzyme-drug-DNA triplet complexes with enzyme-DNA fragmentation complexes, resulting in irreversible fragmentation of DNA fragments. Once these permanent breaks are genetically generated, they become targets for genetic recombination and repair, stimulating the exchange of sister chromosomes in the progeny, and the insertion and deletion of large segments of genes eventually lead to chromatin translocation and aberration, leading to apoptosis.
Camptothecin is a plant alkaloid that targets Top I. Clinical development of camptothecin was discontinued because of its intolerable adverse effects and low therapeutic index. However, derivatives of camptothecin, topotecan and irinotecan, are currently used in the clinic. Unfortunately, their clinical use is limited due to their dose-limiting toxicity, especially neutropenia, myelosuppression, and diarrhea. Rubitecan, under clinical trials, is an orally available camptothecin analogue that also has potential for delivery transdermally or by inhalation. Non-camptothecin Top I inhibitors, such as indolocarbazoles (NB-506), indenoisoquinolines, and dibenzonaphthyridinones (ARC-111) are under clinical development[7].
Some floxacin antibiotics target Top II to exert antibacterial activity, such as Rufloxacin, Doxorubicin, Moxifloxacin, Novobiocin and Gatifloxacin, etc. Inactivation of Top II can also be achieved by restricting the nucleotide-dependent structural changes or inhibiting ATPase activity. ICRF-187 (dexrazoxane) inhibits eukaryotic Top II by arresting the two ATPase domains in the ATP-bound, dimerized form. Epipodophyllotoxins, like Podophyllotoxin, Etoposide, Teniposide are Top II inhibitors, they are widely used in the treatments of solid tumors[7][8]. Because topoisomerase inhibitors have good antitumor and antibacterial activities, there are also many clinical drugs targeting topoisomerase. The table below summarizes topoisomerase inhibitors in clinical trials.
Table 1. Topoisomerase Inhibitors in Clinical Trials
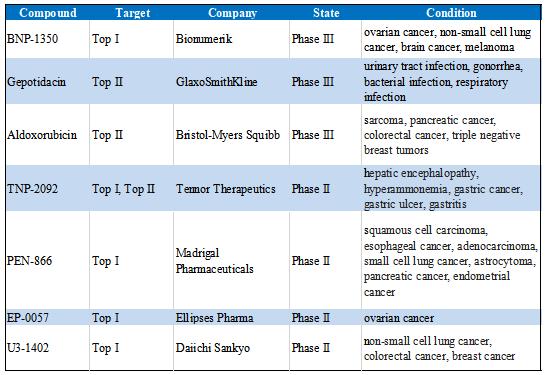
Topoisomerase inhibitors are important targets for tumor chemotherapy, but they still have the problems of excessive toxicity and drug resistance, and most of them only target Top I or Top II, which leads to a decrease in clinical therapeutic effect. Therefore, future research can focus on developing more reasonable Top I/Top II dual inhibitors to improve drug efficacy and reduce toxic side effects. As a leading global supplier of small molecule compounds, CSNpharm has a variety of topoisomerase inhibitors, which can be used for topoisomerase mechanism research and drug discovery. In addition, there is also Anti-cancer Compound Library, which contains 1203 anticancer compounds, which can be used for high-throughput or high-content screening, which are useful for tumor mechanism research, anti-tumor drug screening and drug structure optimization or discovery of new drug targets.
References
[1]https://geneticeducation.co.in/dna-topology-function-of-topoisomerase-1-and-2/
[2]Bush N.G., Evans-Roberts K., Maxwell A. DNA topoisomerases. EcoSal Plus. 2015;6(2).
[3]Pommier Y., Sun Y., Huang S.-Y.N., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016 Nov;17(11):703–721.
[4]Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–40.
[5]Mckie S J , Neuman K C , Maxwell A . DNA topoisomerases: Advances in understanding of cellular roles and multi‐protein complexes viastructure‐function analysis[J]. BioEssays, 2021.
[6]Giles GI, Sharma RP. Topoisomerase enzymes as therapeutic targets for cancer chemotherapy. Med Chem. 2005 Jul;1(4):383-94.
[7]Delgado JL, Hsieh CM, Chan NL, Hiasa H. Topoisomerases as anticancer targets. Biochem J. 2018 Jan 23;475(2):373-398.
[8]Hevener K, Verstak TA, Lutat KE, Riggsbee DL, Mooney JW. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin B.2018 Oct;8(6):844-861.