BLD Insights
25.10.2021
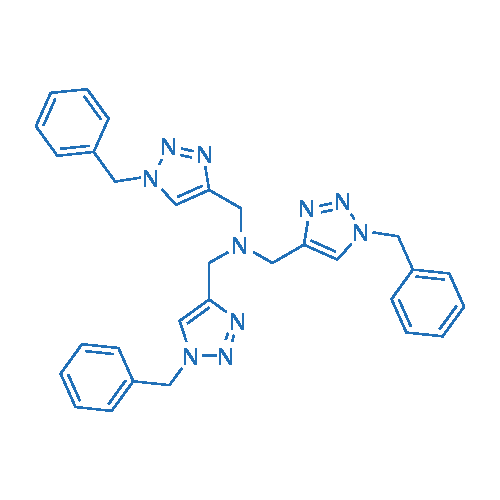
Tris((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine
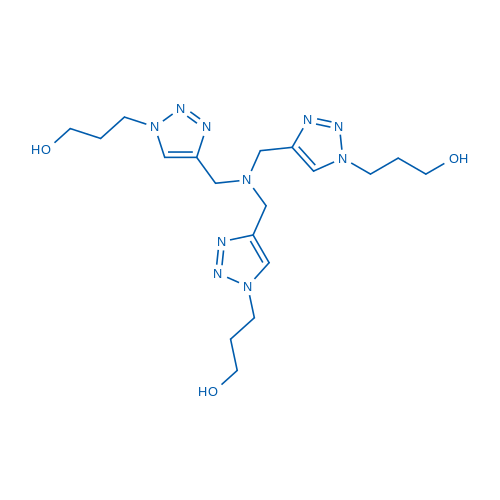
Tris(3-hydroxypropyltriazolylmethyl)amine
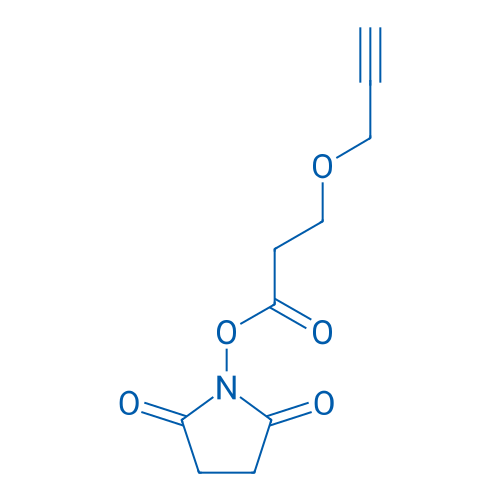
N-Succinimidyl 3-(propargyloxy)propionate
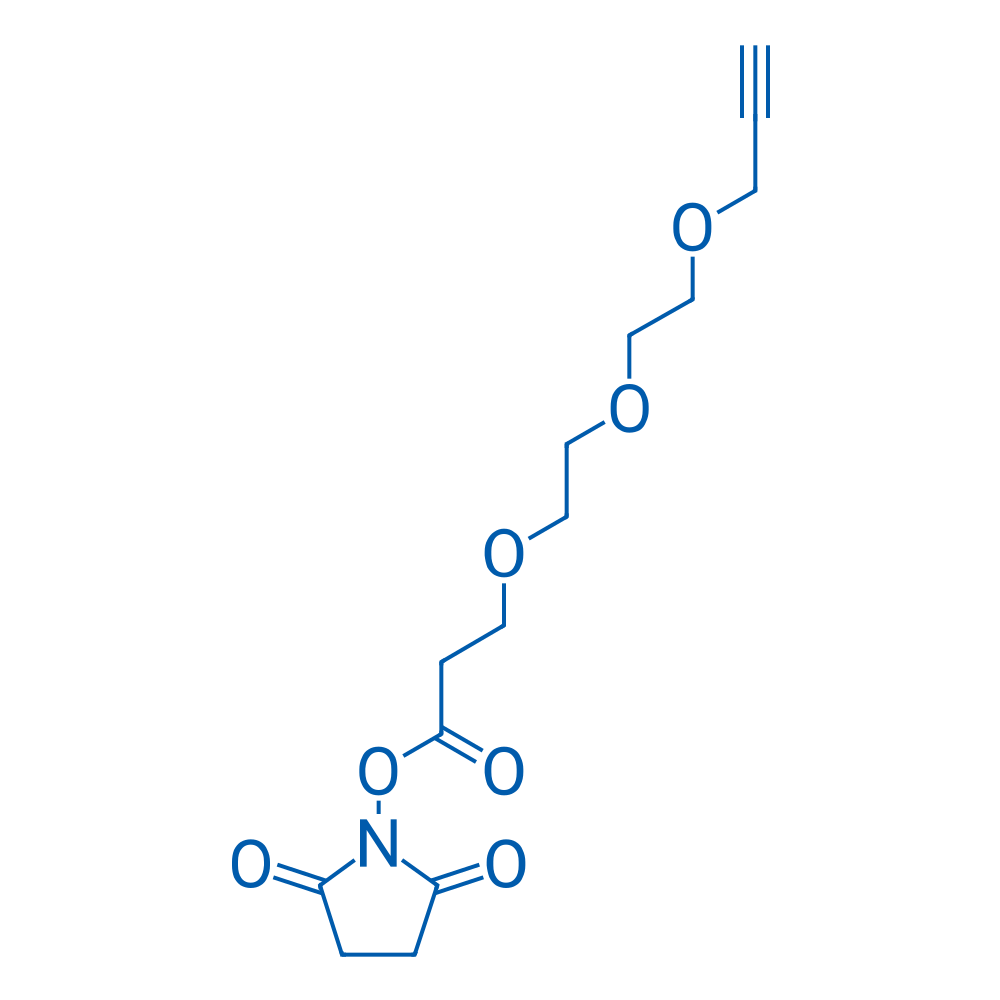
2,5-Dioxopyrrolidin-1-yl 3-(2-(2-(prop-2-yn-1-yloxy)ethoxy)ethoxy)propanoate
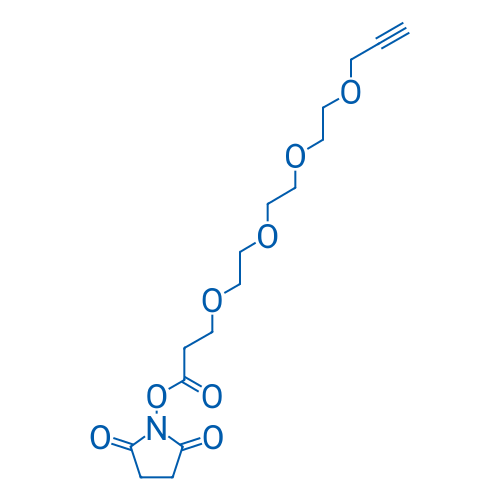
2,5-Dioxopyrrolidin-1-yl 4,7,10,13-tetraoxahexadec-15-yn-1-oate
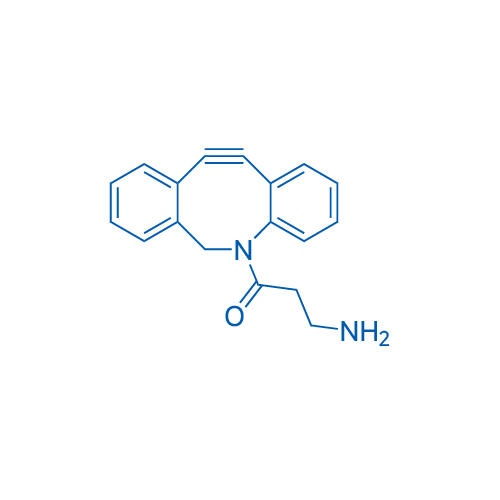
3-Amino-1-(11,12-didehydrodibenz[b,f]azocin-5(6H)-yl)-1-propanone
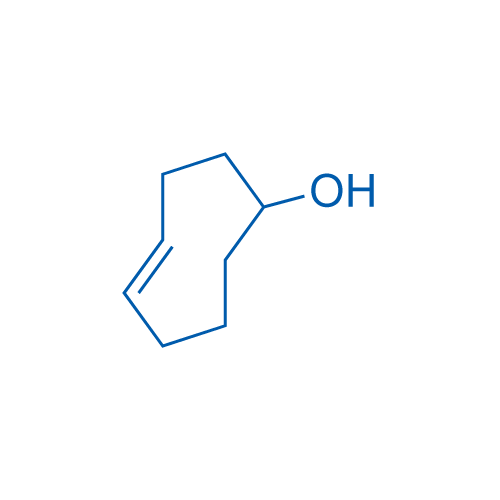
(E)-Cyclooct-4-enol
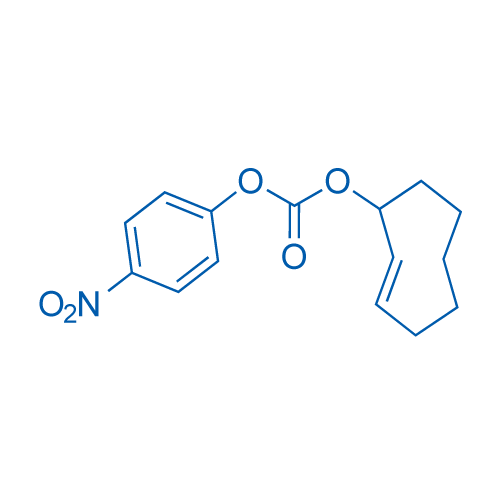
(E)-Cyclooct-2-en-1-yl (4-nitrophenyl) carbonate
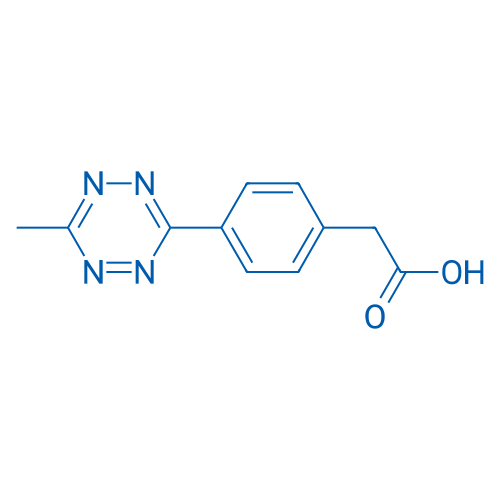
Methyltetrazine-acid
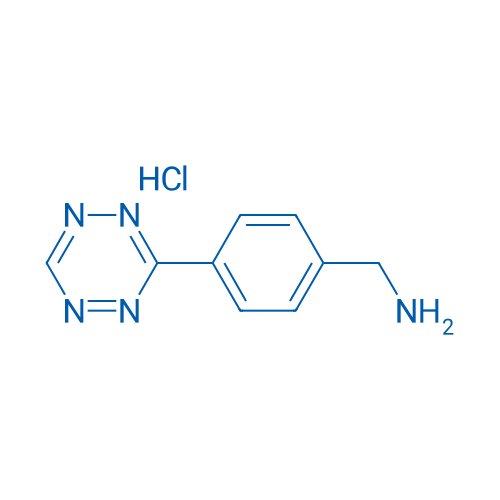
(4-(1,2,4,5-Tetrazin-3-yl)phenyl)methanamine hydrochloride
The "click" concept, proposed by Sharpless, Kolb, and Finn in 2001 (HC Kolb et al., 2001), refers to energetically favored, specific, and versatile chemical transformations, which lead to a single reaction product. The essence of "click" chemistry is simplicity and efficiency. In this context, the straightforward "click" reactions have become tremendously popular in both academic and industrial research, such as molecular biology, drug design, biotechnology, polymer chemistry or materials science.
Copper-Catalyzed Azide–alkyne cycloaddition (CuAAC)
In early 2002, Meldal and co-workers reported that the use of catalytic amounts of copper(I), which can bind to terminal alkynes, leads to fast, highly efficient, and regioselective azide-alkyne cycloadditions at room temperature in organic medium (C.W. Tornoe et al., 2002) (Figure 1). Shortly after, Sharpless and Fokin demonstrated that CuAAC can be successfully performed in polar media, such as tertbutyl alcohol, ethanol or pure water (V.V. Rostovtsev et al., 2002). These two important breakthroughs made such reactions can be performed under experimental conditions compatible with biological environments (e.g. aqueous medium and body temperature). Moreover, azide and alkyne functions are, respectively, absent or relatively rare in the biological world. Thus, azide-alkyne chemistry constitutes a very interesting chemoselective platform for the functionalization or ligation of biological systems (Jean-François Lutz, 2008).
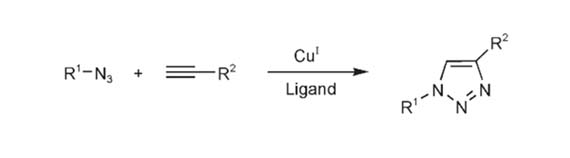
Figure 1. Copper-Catalyzed Azide–Alkyne Cycloaddition
However, the main limitation of CuAAC is the toxicity of Cu(I), which is caused by Cu(I)-mediated generation of reactive oxygen species (ROS) from O2. The use of Cu(I) ligands not only solves this problem but also increases the reaction rate. The first generation of Cu(I) ligand TBTA is the most commonly used, but its cytotoxicity and poor water solubility limit its application. Therefore, a series of ligands such as THTPA, BTTAA, and BTTP have been subsequently developed to increase water solubility and reduce cytotoxicity (Figure 2).
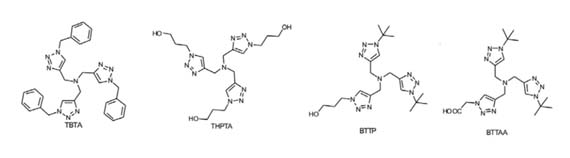
Figure 2. Cu(I) Ligands of CuAAC
Copper-Free Click Chemistry
Strain-promoted azide-alkyne cycloadditions (SPAAC) is another approach to accelerate the azide-alkyne cycloaddition. In 2007, Bertozzi and his co-workers reported a click reaction between cyclooctyne and phenyl azide without metal ion catalysis, which is a consequence of the geometrical deformation of the alkyne bond arising from ring strain (J. M. Baskin et al., 2007). In 2010, Boons and his co-workers reported the SPAAC reaction of dibenzocyclooctyne (DBCO) with azide. Compared with cyclooctyne, DBCO has a greater ring strain, so the reaction rate is extremely Improved (CR Bertozzi et al., 2010); In the same year, Delft and his co-workers reported the SPAAC reaction of bicyclo[6,1,0]nonyne (BCN) with azide. Although the reaction rate is low compared with DBCO, BCN has a smaller size, lower lipophilicity and simpler synthetic route, which make it superior to DBCO in some cases (Jan Dommerholt et al., 2010).
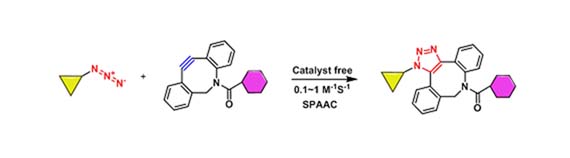
Figure 3. SPAAC reaction of dibenzocyclooctyne (DBCO) with azide
![Figure 4. SPAAC reaction of bicyclo[6,1,0]nonyne (BCN) with azide](/static/img/events_news/news-20211025/BLD4.jpg)
Figure 4. SPAAC reaction of bicyclo[6,1,0]nonyne (BCN) with azide
In addition to the SPAAC reaction of alkyne and azide, the Inverse electron demand Diels-Alder(iEDDA) reaction of transcyclooctene (TCO) and S-tetrazine (Tetrazine) is also widely used bio-orthogonal reactions. This reaction is the fastest bioorthogonal reaction found so far. In addition to the 4E-TCO that can be used in the bioorthogonal ligation reaction, this series of 2E-TCO has also been successfully used in the bioorthogonal cleavage reaction (Melissa L. Blackman et al., 2008).
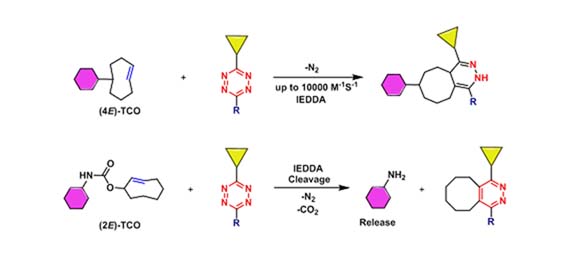
Figure 5. iEDDA reaction of trans-cyclooctene (TCO) and S-tetrazine (Tetrazine)
References
[1] H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056 – 2075; Angew. Chem. Int. Ed. 2001, 40, 2004 – 2021.
[2] A. Michael, J. Prakt. Chem. 1893, 48, 94.
[3] R. Huisgen, Angew. Chem. 1963, 75, 604 – 637; Angew. Chem. Int. Ed. Engl. 1963, 2, 565 – 598.
[4] C. W. Tornoe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057 – 3064.
[5] V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708 – 2711; Angew. Chem. Int. Ed.2002, 41, 2596 – 2599.
[6] Friscourt F, Fahrni C J, Boons G J, J. Am. Chem. Soc. 2012, 134(45):18809-18815.
[7] J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2007, 104, 16793 – 16797.
[8] Jewett J C, Sletten E M, Bertozzi C R, J. Am. Chem. Soc. 2010, 132(11):3688-3690.
[9] Dommerholt J, Schmidt S, Temming R, et al., Angew. Chem. 2010, 122(49):9612-9615.
[10] Blackman M L, Royzen M, Fox J M, J. Am. Chem. Soc. 2008, 130(41):13518-13519.