BLD Insights
Chiral Oxazoline - Containing Ligands
21 February 2023
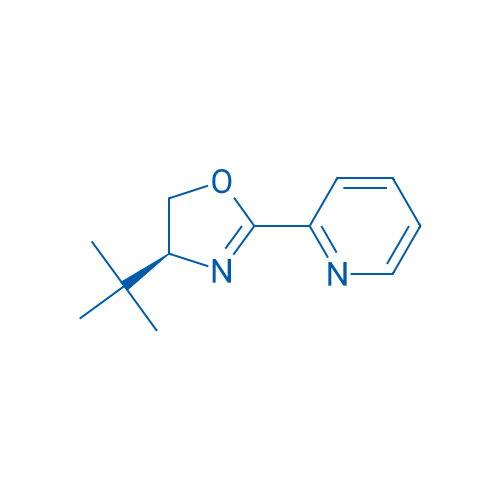
(S)-4-tert-Butyl-2-(2-pyridyl)oxazoline
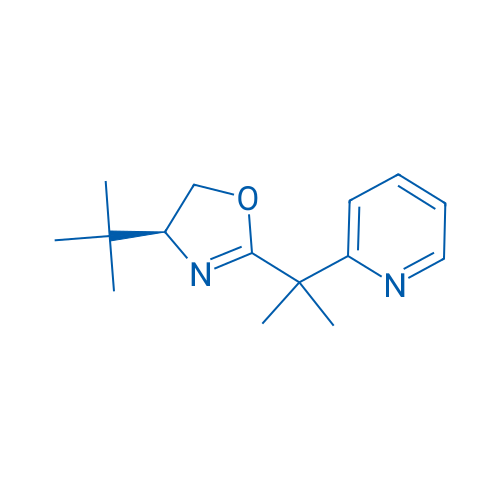
(S)-4-(tert-Butyl)-2-(2-(pyridin-2-yl)propan-2-yl)-4,5-dihydrooxazole
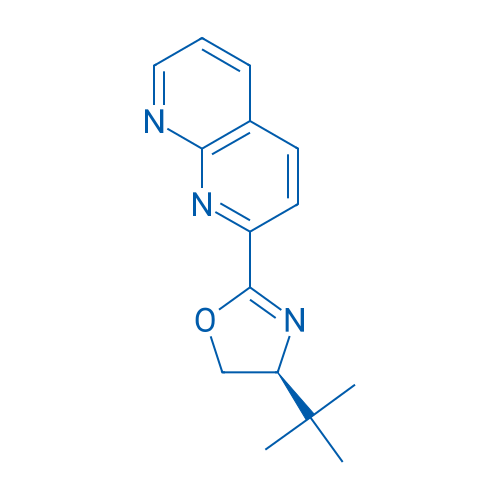
(S)-4-(tert-Butyl)-2-(1,8-naphthyridin-2-yl)-4,5-dihydrooxazole
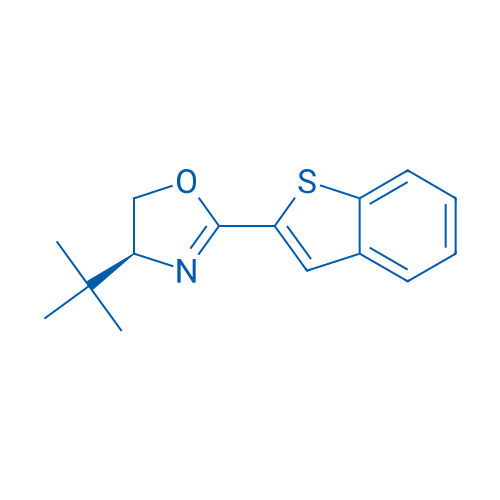
(S)-2-(Benzo[b]thiophen-2-yl)-4-(tert-butyl)-4,5-dihydrooxazole
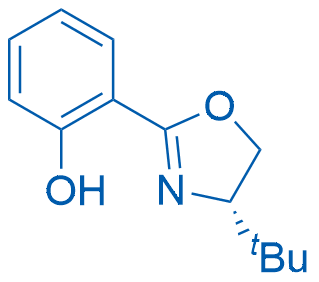
(S)-2-(4-(tert-Butyl)-4,5-dihydrooxazol-2-yl)phenol
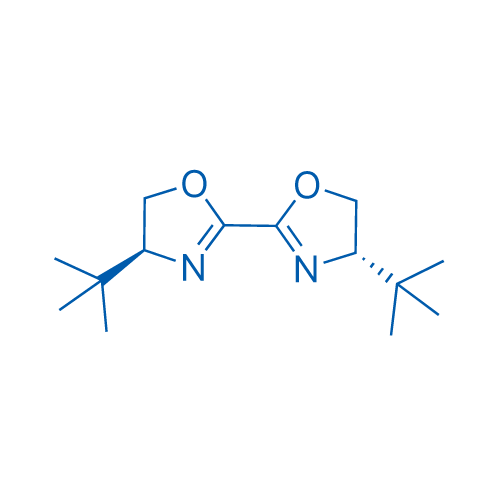
(4S,4'S)-4,4'-Bis(1,1-dimethylethyl)-4,4',5,5'-tetrahydro-2,2'-bioxazole
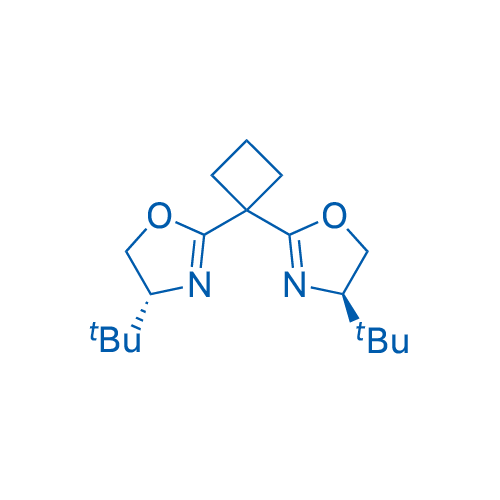
(4R,4'R)-2,2'-(Cyclobutane-1,1-diyl)bis(4-(tert-butyl)-4,5-dihydrooxazole)
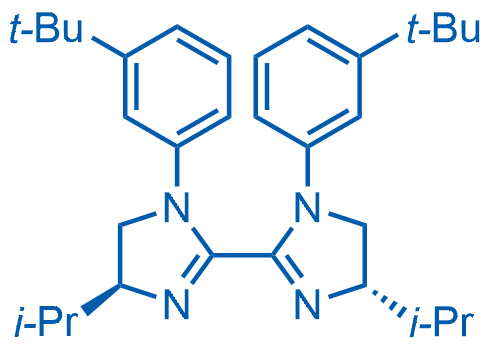
(4S,4'S)-1,1'-Bis(3-(tert-butyl)phenyl)-4,4'-diisopropyl-4,4',5,5'-tetrahydro-1H,1'H-2,2'-biimidazole
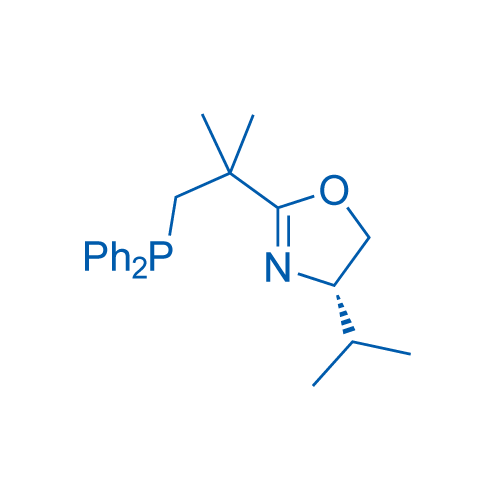
(S)-2-(1-(Diphenylphosphanyl)-2-methylpropan-2-yl)-4-isopropyl-4,5-dihydrooxazole
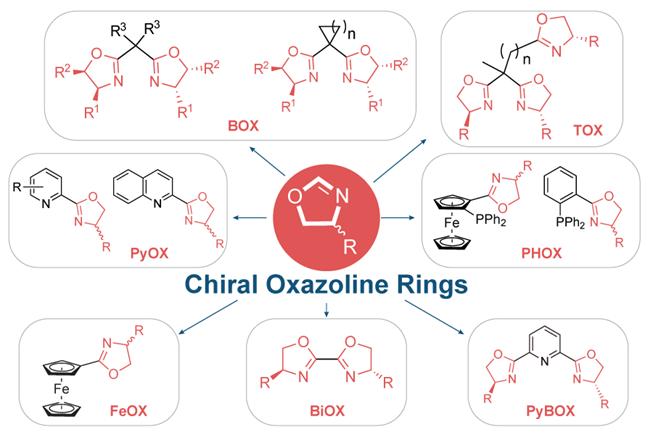
Asymmetric catalysis with chiral metal catalysts has received considerable attention in recent years, and its contribution to organic synthesis has become of leading importance. The chiral metal catalyst, in general consists of a metal active site coordinating with a chiral organic ligand, among these various ligands, chiral oxazoline-containing ligands are one of the most successful and commonly used classes of ligands due to their ready accessibility, modular nature, and extensive applicability in metal-catalyzed asymmetric reactions with excellent catalytic activity and enantioselectivity.
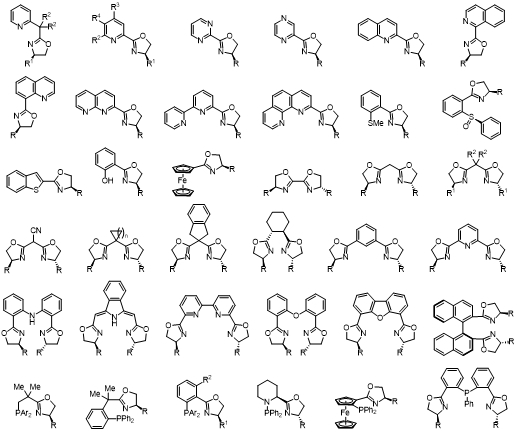
These ligands are derived from readily available chiral amino alcohols, in short, high-yielding synthetic sequences. The enantiostereocenter resides on the carbon atom, neighboring the coordinating nitrogen of the oxazoline ring which is close to the metal active site, thus having a direct influence on the stereochemical effect of the reaction.
Technical Notes:
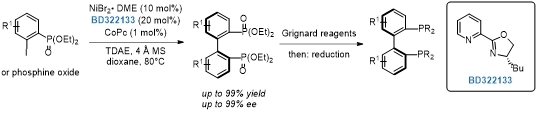
Synthesis of axially chiral 2,2′-bisphosphobiarenes via a nickel-catalyzed asymmetric Ullmann coupling.[1]
Three-component asymmetric Ni-catalyzed 1,2-dicarbofunctionalization of unactivated alkenes.[2]
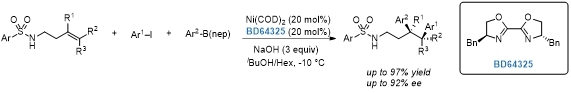
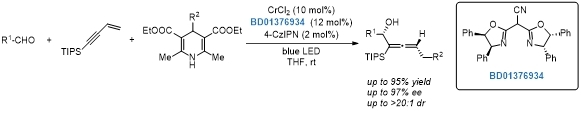
Chromium/photo-cocatalyzed asymmetric 1,4-functionalization of 1,3-enynes.[3]
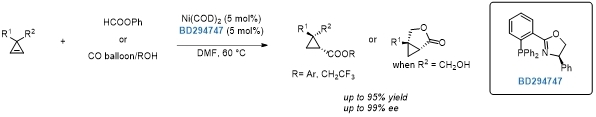
Nickel-catalyzed asymmetric hydroaryloxy- and hydroalkoxycarbonylation of cyclopropenes.[4]
References
[1]J. Am. Chem. Soc. 2021, 143, 1328.
[2]J. Am. Chem. Soc. 2022, 144, 19337.
[3]Nat. Commun. 2022, 13, 5036.
[4]Angew. Chem. Int. Ed. 2022, 10.1002/anie.202200733.