How BLD Helps
New Carbon-Expanding Reagents: Streamlining the Synthesis of Ambrettolide from 14 Steps to Just 2 Steps
30 May 2025
Homologation reactions are crucial tools in organic synthesis for the fine-tuning of carbon skeletons, enabling the introduction of a single carbon unit while preserving existing functional groups. These reactions are widely used in the construction of drug molecules and natural products.
We know that carboxylic acids are common structural units in pharmaceutical intermediates, and their single-carbon chain extension shows significant potential in the design of β-amino acids, drug analogs, and complex molecule synthesis. However, achieving direct homologation of carboxylic acids remains challenging. Current methods often rely on reactive intermediates such as acyl chlorides or thioesters, involving complex steps and typically requiring hazardous reagents like diazomethane.
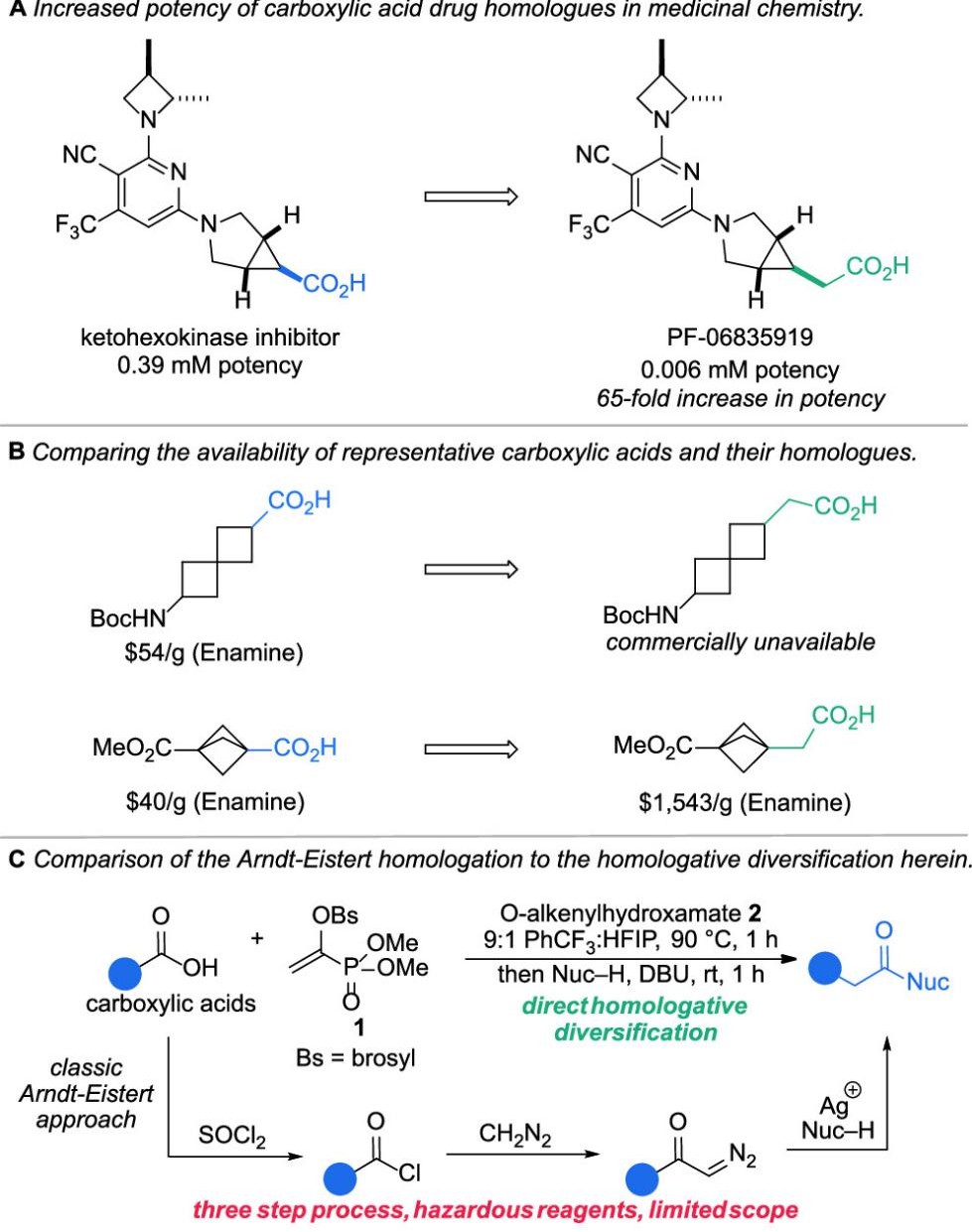
Three step process, hazardous reagents, limited scope
Therefore, developing a mild, simple, and efficient method for the single-carbon expansion of carboxylic acids is of great importance for accelerating molecular optimization and new drug development.
This article introduces a one-step carbon-expansion strategy based on natural carboxylic acids, utilizing a novel and stable (1-phosphoryl)vinyl sulfonate reagent (1), which enables efficient homologation of carboxylic acids under mild conditions.
The method is applicable to various aliphatic carboxylic acids and structurally complex bioactive molecules, allowing for the extension of carbon chains by a single -CH₂- unit while retaining functional groups, and generating a range of homologated products such as esters, amides, and carboxylic acids.
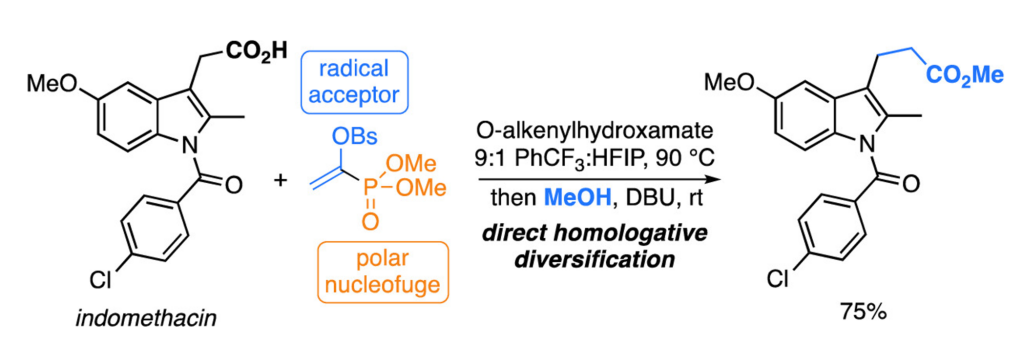
(1-Phosphoryl)vinyl sulfonate (1) is a dual-functional organic reagent that ingeniously combines two reactive sites: radical trapping and electrophilic activity. The vinyl sulfonate moiety selectively captures the carbon-centered radical generated from decarboxylation of carboxylic acids, while the phosphoryl group can be transformed into an active acyl intermediate, which further participates in nucleophilic substitution reactions. The introduction of the p-bromosulfonyl (Brosyl) group into the reagent enhances overall crystallinity and air stability, making it easy to weigh and store for long-term use in laboratory settings.
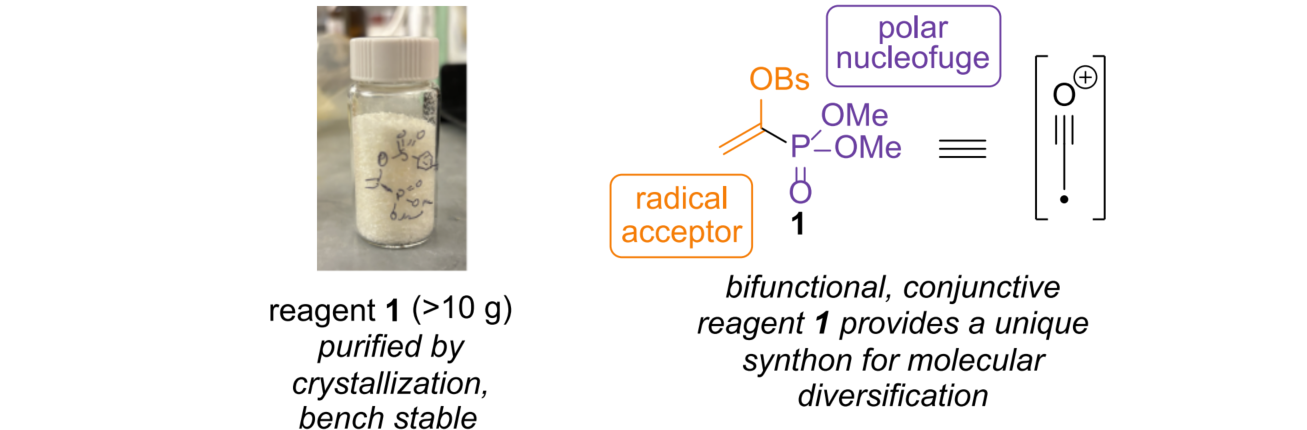
This molecular design not only ensures high reaction efficiency but also provides a platform for generating distonic acylium radicals, a novel class of synthetic intermediates. Compared with traditional methods such as the Arndt–Eistert or Barton reactions, this approach eliminates tedious intermediate conversion steps and avoids the use of highly toxic or reactive reagents, demonstrating great potential for rapid modification and diversity-oriented synthesis of drug molecules.
This method enables carboxylic acid homologation through two possible mechanisms.
The first mechanism occurs under thermal conditions, where O-alkyl oxime esters decompose to generate amidyl radicals. These radicals induce the carboxylic acid to undergo hydrogen transfer and decarboxylation, producing an alkyl radical. This radical then adds to the vinyl sulfonate moiety, forming a phosphodiester intermediate. Finally, under basic conditions, the intermediate reacts with a nucleophile to yield the target homologated product.
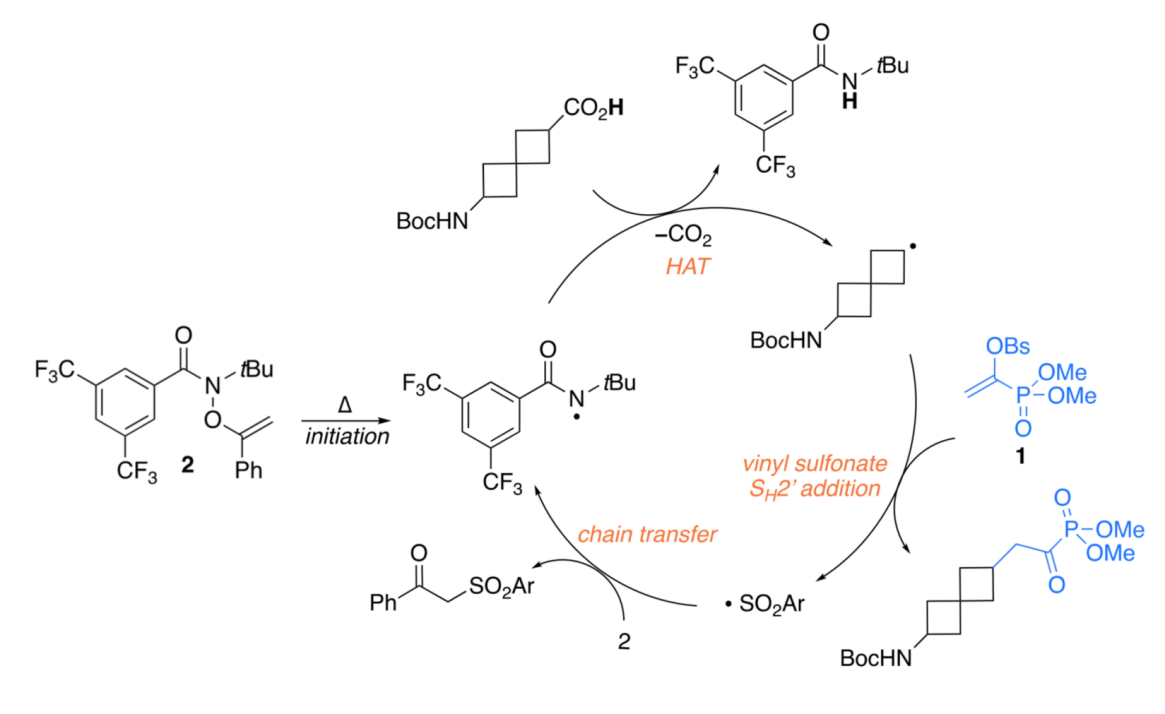
The second pathway employs visible light and an acridine-based photocatalyst to initiate a single-electron transfer (SET) oxidation of the carboxylic acid, triggering a similar radical transformation. This approach is particularly suitable for thermally sensitive substrates.
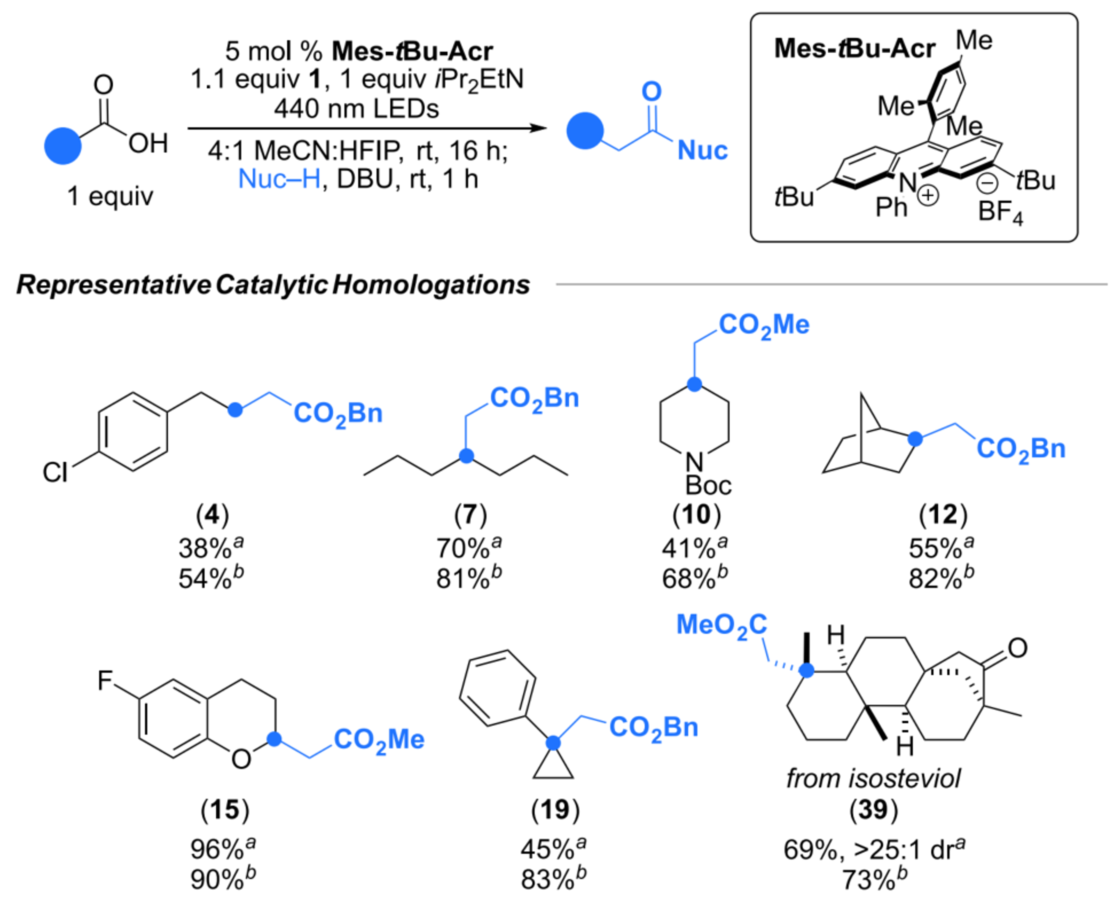
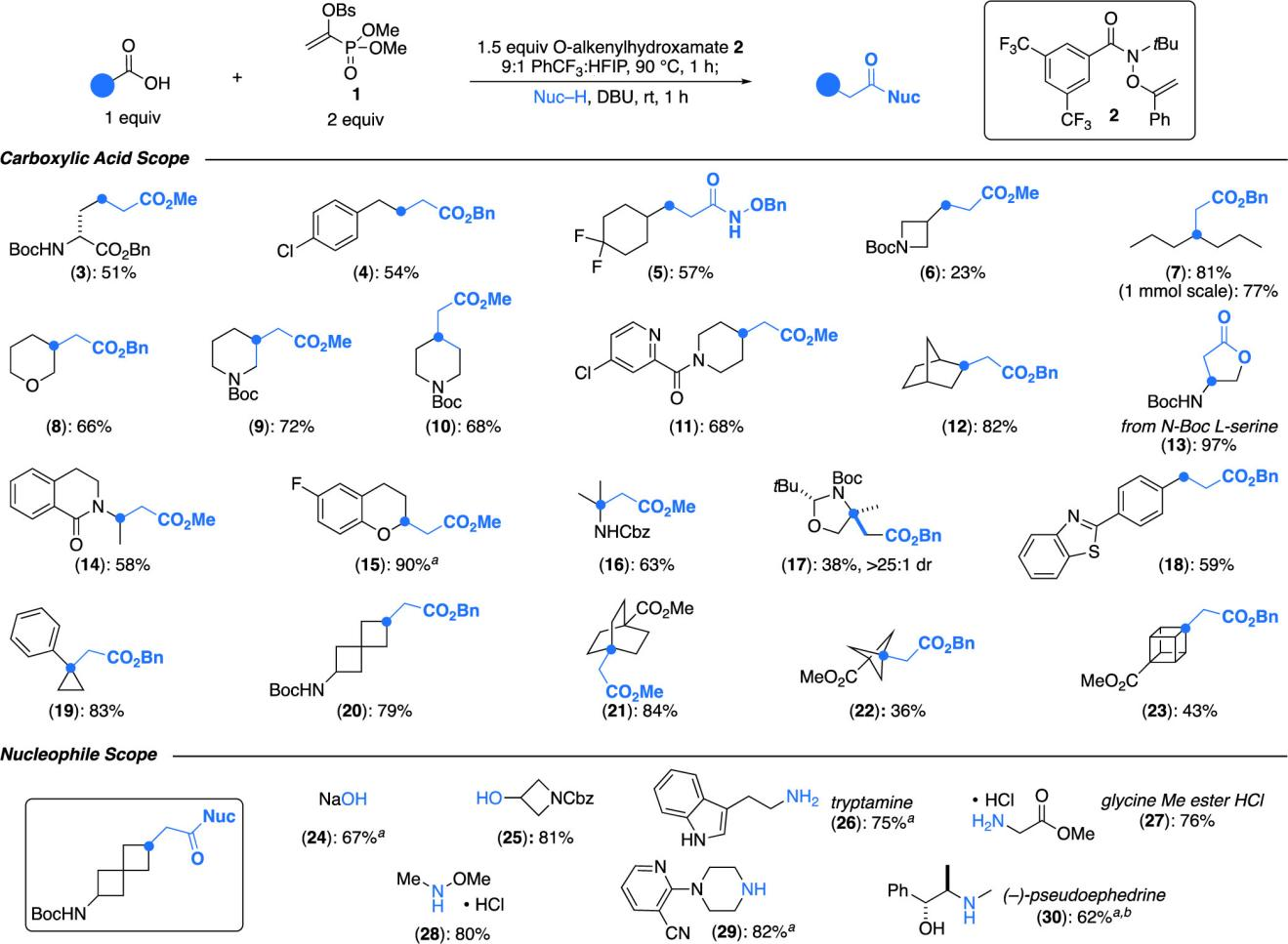
This reagent is compatible with a wide range of carboxylic acid substrates, including primary, secondary, and tertiary aliphatic acids, as well as heterocyclic and strained-ring structures. It also tolerates various nucleophiles such as alcohols, amines, and water.
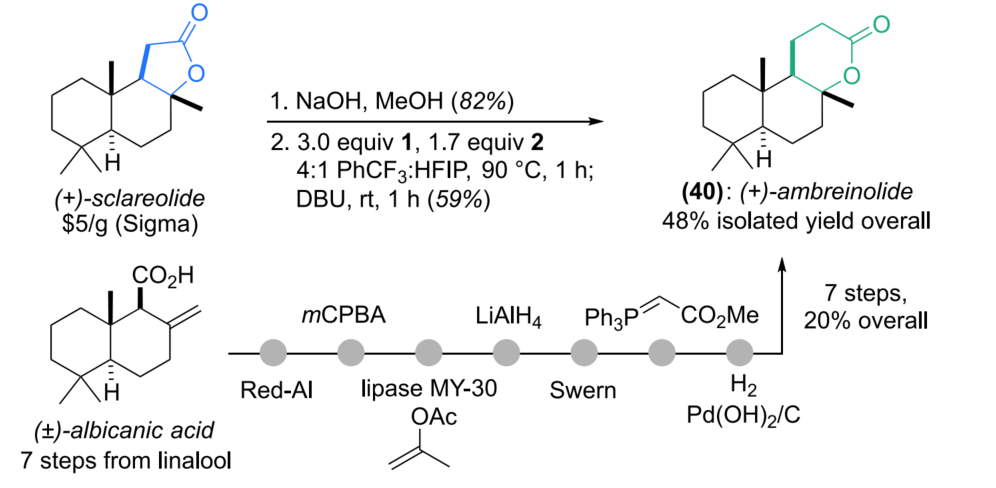
In the synthesis of (+)-Ambreinolide, the new method efficiently constructs the target molecule in just two steps — hydrolysis followed by homologation/cyclization — with an overall yield of 48%. Compared to the traditional synthetic route, which requires 14 steps starting from linalool and suffers from low overall yield, this strategy significantly simplifies the synthetic process and greatly improves the overall efficiency.
This novel (1-phosphoryl)vinyl sulfonate (1) reagent enables one-step homologation of carboxylic acids under mild conditions. Combining both radical and polar reaction features, the method demonstrates broad substrate scope and versatility in nucleophile compatibility, showcasing particular advantages in the rapid synthesis of natural products and drug molecules.
PRODUCT SUGGESTION
Click on the structure to check the details.
References
[1]J. Am. Chem. Soc. 2024, 146, 32919−32924.