How BLD Helps
Sulfur(VI) Fluoride Exchange (SuFEx): The "Golden Bond" for Next-Generation Click Chemistry — A High-Efficiency, Stable, and Biocompatible Ligation Technology
23 May 2025
Click Chemistry, introduced by Nobel Laureate in Chemistry K. Barry Sharpless in 2001, refers specifically to a class of highly efficient, highly selective, and modular chemical reactions. Its core characteristics include: 1/ High efficiency: yields are typically greater than 90%; 2/ Biological orthogonality: reactions can safely proceed in physiological environments; 3/ Modular construction capability: akin to "molecular Lego," it enables the rapid assembly of complex structures. Among these, the new generation of click chemistry technology, sulfur (VI) fluoride exchange (SuFEx), with its unique reaction properties, is bringing new ideas to fields such as drug development and proteomics research.
Part 1: Advantages of SuFEx Reaction [1]
a) Resistance to Oxidation and Reduction
Due to the strong electronegativity of fluorine, the electron cloud is heavily biased towards F, causing the cleavage of the sulfonyl fluoride bond to be entirely heterolytic, without generating active radical intermediates and with a high bond energy that makes it difficult to break. In contrast, homolytic cleavage of S-Cl bonds is quite common. This characteristic allows it to remain stable in reductive biological environments, such as those rich in glutathione (GSH), making it an ideal choice for labeling live cells and in vivo applications.
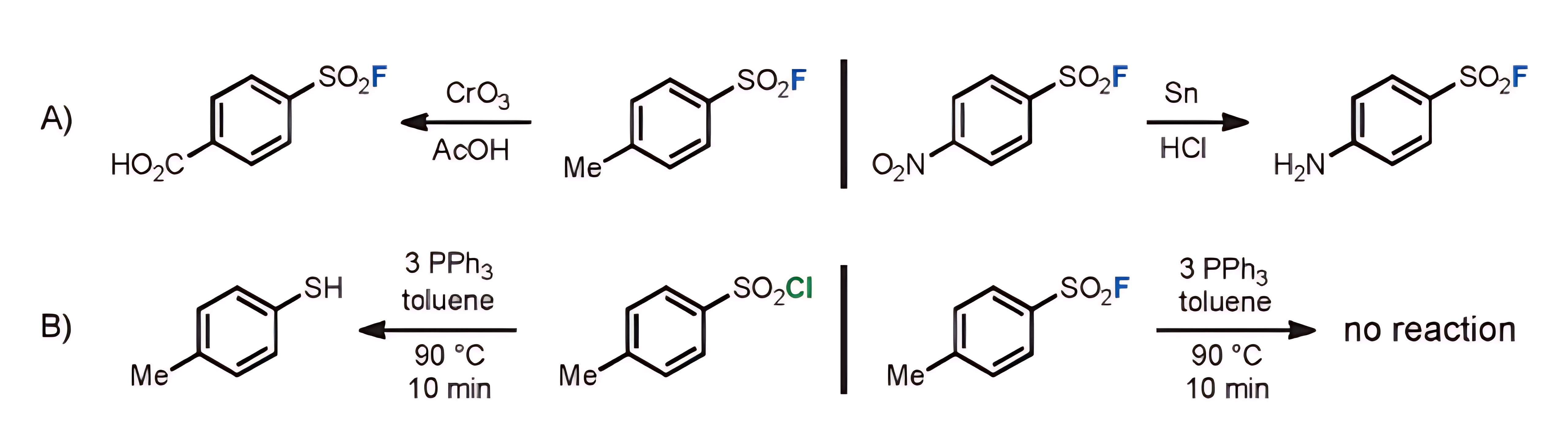
b) Thermodynamic Stability
Sulfonyl fluorides exhibit greater stability than other sulfonyl halides in nucleophilic substitution reactions, including hydrolysis, as well as in thermal decomposition reactions. The homolytic bond dissociation energy of the S-F bond in SO2F2 (90.5±4.3 kcal/mol) is significantly higher than that of the S-Cl bond in SO2Cl2 (46±4 kcal/mol). This substantial difference in bond energy makes sulfonyl fluorides the preferred choice under demanding reaction conditions. Their enhanced stability ensures that they can withstand more extreme conditions without decomposing or reacting undesirably, which is crucial for applications requiring high reliability and specificity.
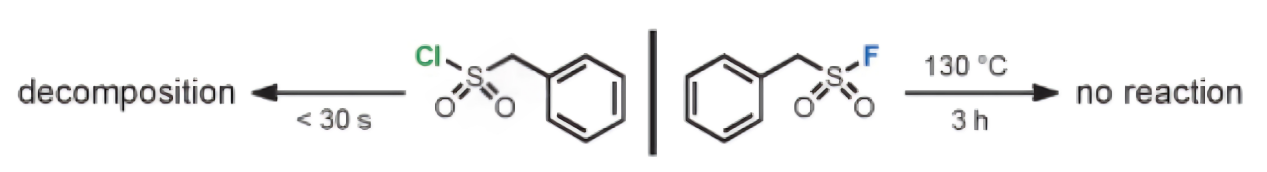
c) Exclusive Reactivity at Sulfur Center
The high polarizability of the chlorine center in -SO2Cl and related groups makes it susceptible to reductive attack, which can lead to homolytic cleavage and the generation of the radical Cl·. Given its electrophilic nature, Cl· can result in chlorinated products. Consequently, reactions of -SO2Cl with carbon nucleophiles typically yield a mixture of sulfonated and chlorinated products. In contrast, sulfonyl fluorides under these circumstances produce only sulfonated outcomes. This distinctive characteristic makes sulfonyl fluorides an excellent tool for precise chemical modifications, ensuring that the desired sulfonation occurs without unwanted side reactions such as chlorination. This specificity is particularly valuable in complex synthetic scenarios where control over the reaction outcome is critical.
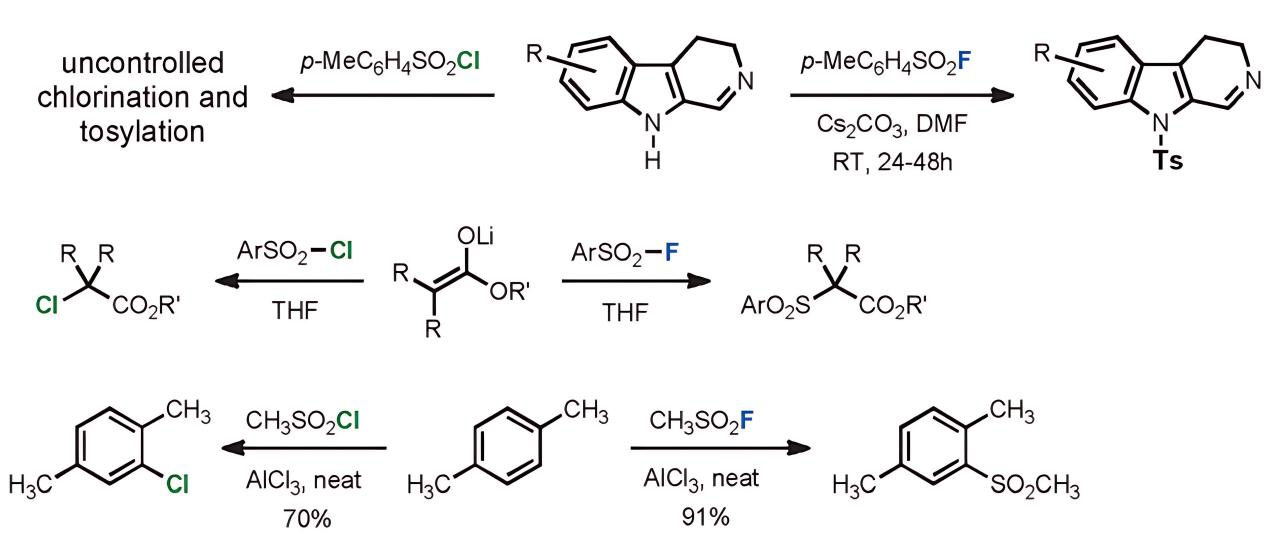
d) Special Properties of Fluoride Ion and Proton Interaction
In the nucleophilic substitution reactions involving sulfonyl fluorides, the stability of the fluoride ion during the substitution process plays a crucial role. Both H+ and R3Si+ can stabilize the fluoride ion, making the SuFEx process controllable and applicable in both biological and synthetic environments. This can be understood as follows: When F- acts as a leaving group, it captures a proton between its two lone pairs to form a symmetric [F-H-F]- ion, which is a 4-electron-3-center bond. This structure enhances stability and suppresses the backward nucleophilic attack by F-. Similarly, when combined with R3Si+, F- forms the stable R3Si-F, driving the reaction completely towards the sulfonation direction.
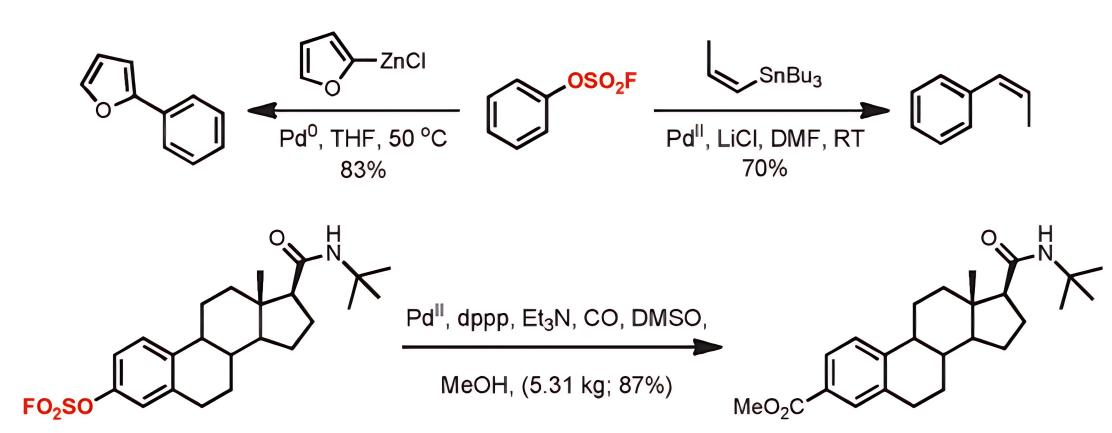
Part 2: Applications of SuFEx
a) Pharmaceutical Chemistry: Transition Metal-Catalyzed Coupling Reactions [1]
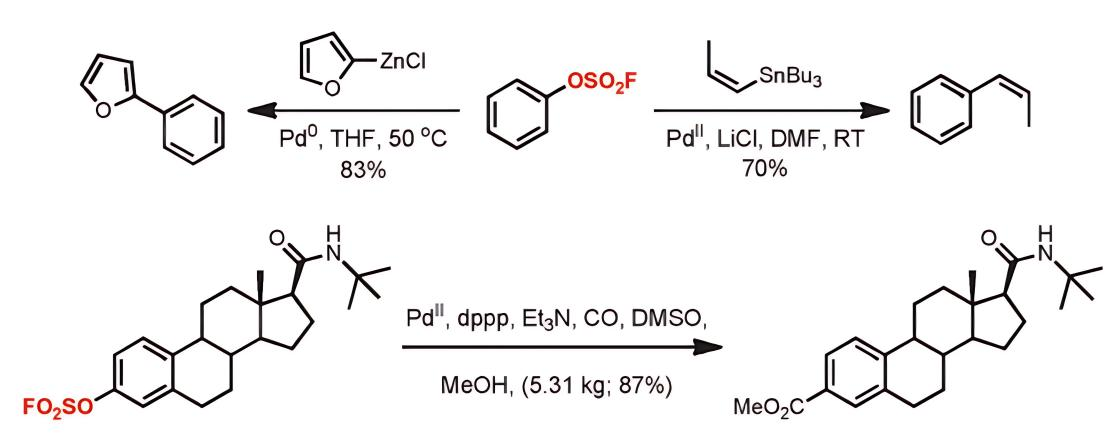
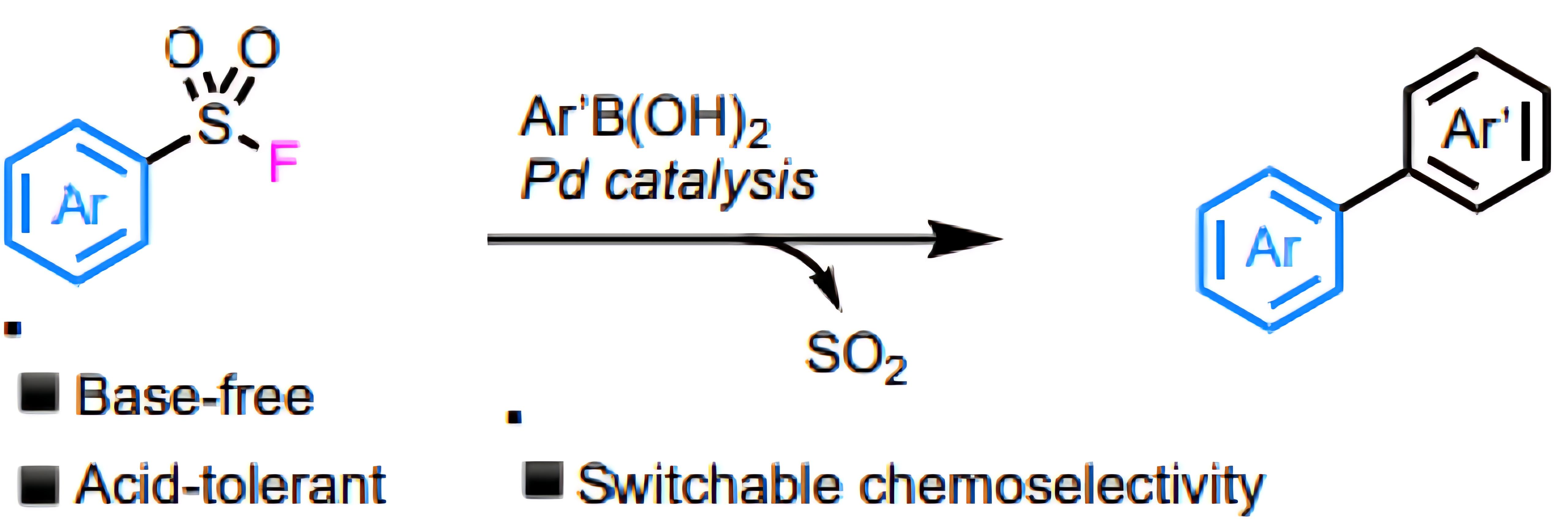
Aromatic sulfonyl fluoride compounds can participate in transition metal-catalyzed coupling reactions. Research by Bristol-Myers Squibb (BMS) [2] has shown that sulfonyl fluorides act as electrophiles in Negishi and Stille cross-coupling reactions, as well as palladium-catalyzed carbonylation reactions. The Joseph Moran group reported the use of sulfonyl fluorides as electrophiles in Suzuki coupling reactions [3], enabling C-C bond formation without a base and under strongly acidic conditions.
b) Drug Development: Design of Covalent Inhibitors
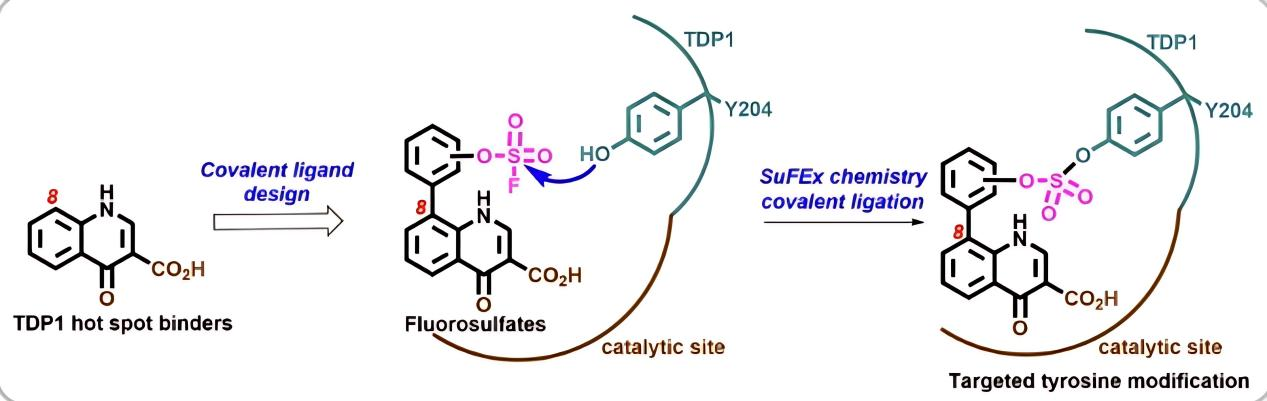
Mainstream covalent modification strategies often target cysteine (Cys), utilizing its high nucleophilicity with electrophilic reagents like maleimides and acrylamides for precise modification or inhibition. However, Cys sites are limited and some are inaccessible due to steric hindrance. Sulfonyl fluoride (SuFEx) reagents exhibit broad reactivity with various nucleophilic amino acids (such as Tyr, Lys, His, Arg, Ser, Thr), forming stable covalent bonds efficiently and selectively. In 2024, NIH research [4] utilized SuFEx technology to develop potent covalent inhibitors targeting tyrosyl-DNA phosphodiesterase 1 (TDP1). Earlier, Baker et al. [5,6] also developed multiple covalent inhibitors using SuFEx, including inhibitors of dihydrofolate reductase (DHFR), guanine deaminase (GAH), α-chymotrypsin, and fatty acid amide hydrolase (FAAH). These studies collectively demonstrate the unique value of SuFEx technology in covalent drug design.
c) Proteomics: Probe Design
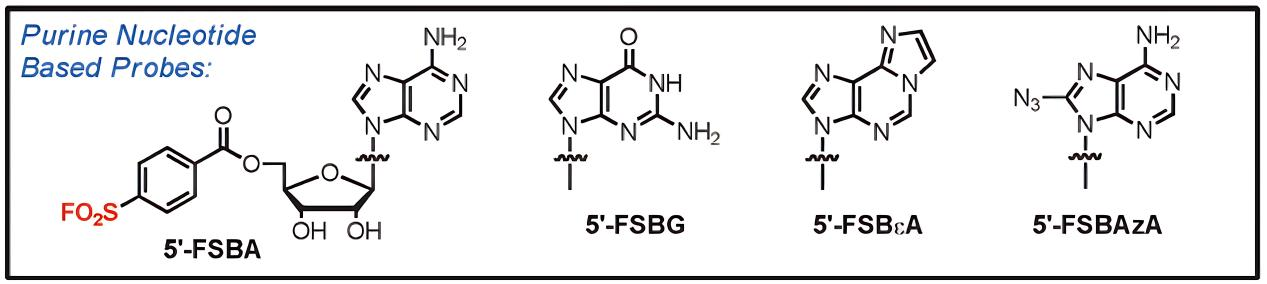
The research team led by Roberta F. Colman at the University of Delaware has developed a series of fluorosulfonylbenzoyl nucleoside probes [7]. Among them, 5'-FSBA can specifically label NAD and ATP binding sites in over 50 proteins; 5'-FSBεA has been developed into a highly sensitive fluorescent affinity probe for kinase activity detection. This sulfonyl fluoride-based probe design strategy has been applied by multiple research groups, including in the inhibition of adenosine receptors and Src family tyrosine kinases, demonstrating the utility of sulfonyl fluorides in studying protein interactions and functional proteomics.
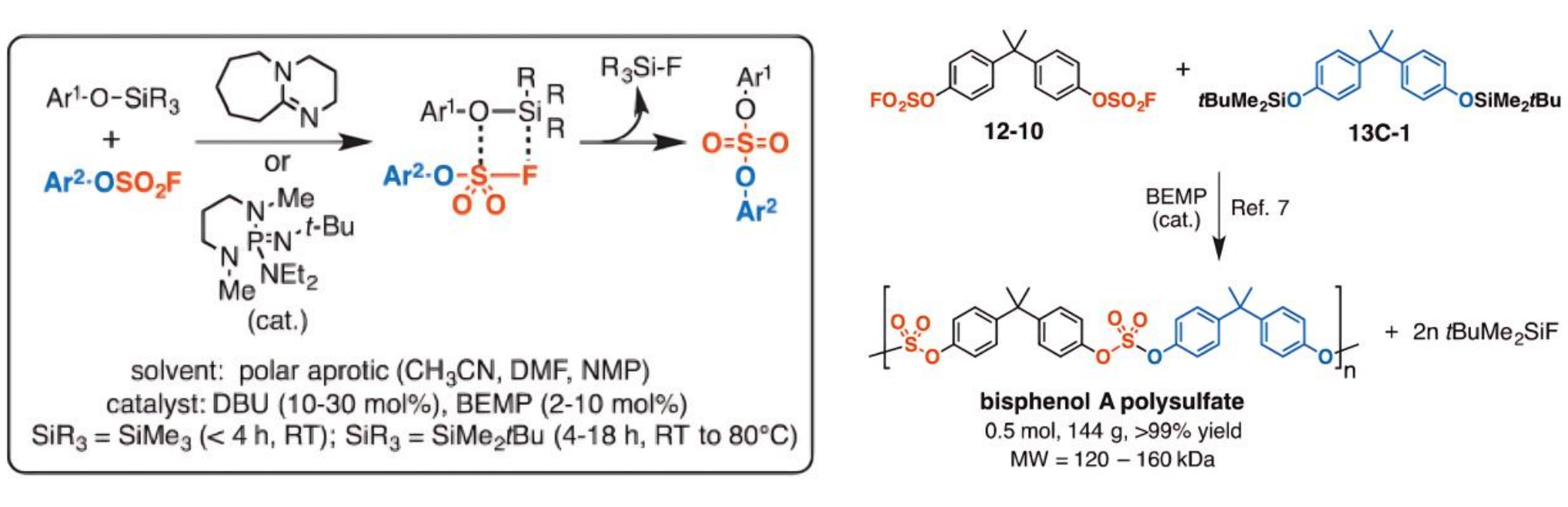
d) Materials Science: Synthesis of Polymeric Materials
Gembus et al. reported [8] that Lewis base-catalyzed coupling reactions between silanes and sulfonyl fluorides can efficiently form stable sulfonate ester bonds with high yields. Key advantages include: 1) High selectivity, producing only inert or even volatile silicon fluoride by-products; 2) Excellent functional group compatibility, limited mainly by the steric hindrance around the silicon atom and interference from acidic protons that could quench the basic catalyst. These features make it an ideal choice for synthesizing polymeric materials, providing new approaches for the molecular design of high-performance materials.
How BLD Pharm helps
Sulfonyl Fluoride Molecular Library
Offering a diverse collection of over 1000 sulfonyl fluoride compounds, including aryl sulfonyl fluorides, alkyl sulfonyl fluorides, sulfonyl fluoride esters, and amino sulfonyl fluorides. Custom synthesis services are also available.
Scale-up Production
Providing scalable process development and production support for sulfonyl fluoride compounds, facilitating a seamless transition from laboratory research to industrial-scale manufacturing.
Featured Product Recommendations
Aryl Sulfonyl Fluorides
Alkyl Sulfonyl Fluorides
Sulfonyl Fluoride Esters
Amino Sulfonyl Fluorides
References
[1]10.1002/anie.201309399
[2]10.1016/0040-4039(92)88113-J
[3]10.1016/S0040-4039(98)01882-6
[4]10.1038/s42004-024-01298-w
[5]10.1021/jo00875a011
[6]10.1021/jm00223a014
[7]10.1096/fasebj.11.4.9068610
[8]10.1002/ange.201403758